|
Iodine-131
Iodine-131 (131I, I-131) is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission (by weight). See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium. Due to its mode of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fission Product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release of heat energy (kinetic energy of the nuclei), and gamma rays. The two smaller nuclei are the ''fission products''. (See also Fission products (by element)). About 0.2% to 0.4% of fissions are ternary fissions, producing a third light nucleus such as helium-4 (90%) or tritium (7%). The fission products themselves are usually unstable and therefore radioactive. Due to being relatively neutron-rich for their atomic number, many of them quickly undergo beta decay. This releases additional energy in the form of beta particles, antineutrinos, and gamma rays. Thus, fission events normally result in beta and additional gamma radiation that begins immediately after, even though this radiation is not produced directly by the fission even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fission Product Yield
Nuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission. Yield can be broken down by: # Individual isotope # Chemical element spanning several isotopes of different mass number but same atomic number. # Nuclei of a given mass number regardless of atomic number. Known as "chain yield" because it represents a decay chain of beta decay. Isotope and element yields will change as the fission products undergo beta decay, while chain yields do not change after completion of neutron emission by a few neutron-rich initial fission products ( delayed neutrons), with half-life measured in seconds. A few isotopes can be produced directly by fission, but not by beta decay because the would-be precursor with atomic number one less is stable and does not decay (atomic number grows by 1 during beta decay). Chain yields do not account for these "shadowed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek , meaning 'violet'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compounds, it has also found favour as a non-toxic radiocontrast material. Because of the spec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiation-induced Cancer
Exposure to ionizing radiation is known to increase the future incidence of cancer, particularly leukemia. The mechanism by which this occurs is well understood, but quantitative models predicting the level of risk remain controversial. The most widely accepted model posits that the incidence of cancers due to ionizing radiation increases linearly with effective radiation dose at a rate of 5.5% per sievert; if correct, natural background radiation is the most hazardous source of radiation to general public health, followed by medical imaging as a close second. Additionally, the vast majority of non-invasive cancers are non-melanoma skin cancers caused by ultraviolet radiation (which lies on the boundary between ionizing and non-ionizing radiation). Non-ionizing radio frequency radiation from mobile phones, electric power transmission, and other similar sources have been investigated as a possible carcinogen by the WHO's International Agency for Research on Cancer, but to date, no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Iodine
There are 40 known isotopes of iodine (53I) from 108I to 147I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element. Its longest-lived radioactive isotope, 129I, has a half-life of 16.14 million years, which is too short for it to exist as a primordial nuclide. Cosmogenic sources of 129I produce very tiny quantities of it that are too small to affect atomic weight measurements; iodine is thus also a mononuclidic element—one that is found in nature only as a single nuclide. Most 129I derived radioactivity on Earth is man-made, an unwanted long-lived byproduct of early nuclear tests and nuclear fission accidents. All other iodine radioisotopes have half-lives less than 60 days, and four of these are used as tracers and therapeutic agents in medicine - 123I, 124I, 125I, and 131I. All industrial use of radioactive iodine isotopes involves these four. The isotope 135I has a half-life less than seven hours, which is inconveniently ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graves' Disease
Graves' disease, also known as toxic diffuse goiter or Basedow's disease, is an autoimmune disease that affects the thyroid. It frequently results in and is the most common cause of hyperthyroidism. It also often results in an enlarged thyroid. Signs and symptoms of hyperthyroidism may include irritability, muscle weakness, sleeping problems, a fast heartbeat, poor tolerance of heat, diarrhea and unintentional weight loss. Other symptoms may include thickening of the skin on the shins, known as pretibial myxedema, and eye bulging, a condition caused by Graves' ophthalmopathy. About 25 to 30% of people with the condition develop eye problems. The exact cause of the disease is unclear, but symptoms are a result of antibodies binding to receptors on the thyroid causing over-expression of thyroid hormone. Persons are more likely to be affected if they have a family member with the disease. If one monozygotic twin is affected, a 30% chance exists that the other twin will ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
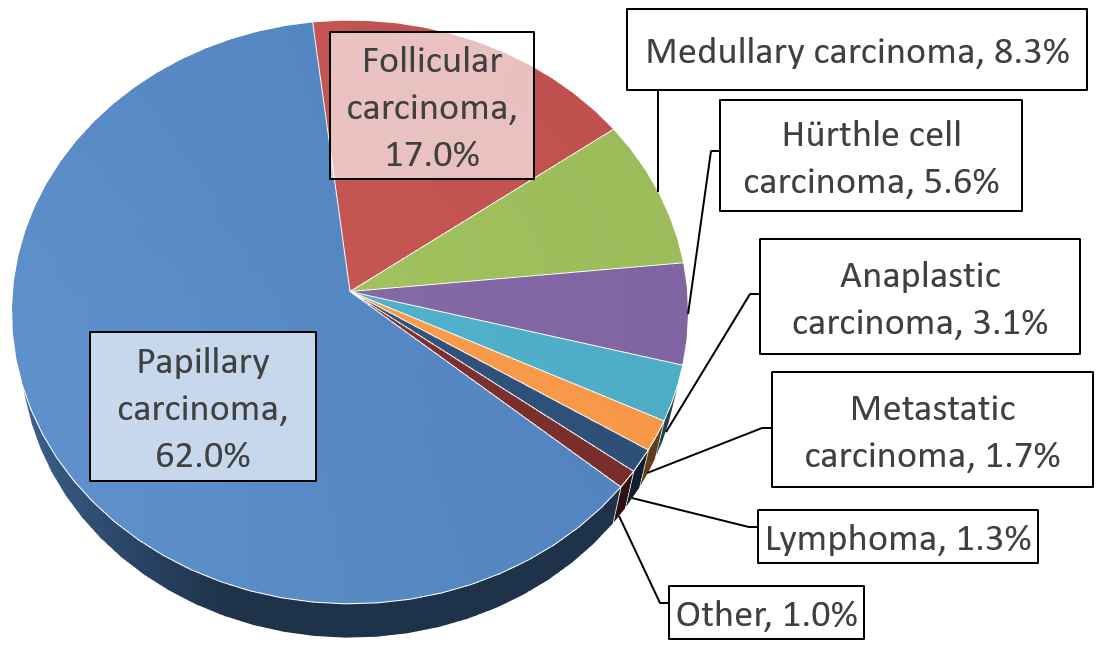
Thyroid Cancer
Thyroid cancer is cancer that develops from the tissues of the thyroid gland. It is a disease in which cells grow abnormally and have the potential to spread to other parts of the body. Symptoms can include swelling or a lump in the neck, difficulty swallowing or voice changes including hoarseness, or a feeling of something being in the throat due to mass effect from the tumor. However, most cases are asymptomatic. Cancer can also occur in the thyroid after spread from other locations, in which case it is not classified as thyroid cancer. Risk factors include radiation exposure at a young age, having an enlarged thyroid, family history and obesity Obesity is a medical condition, considered by multiple organizations to be a disease, in which excess Adipose tissue, body fat has accumulated to such an extent that it can potentially have negative effects on health. People are classifi .... The four main types are papillary thyroid cancer, follicular thyroid canc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Medicine
Nuclear medicine (nuclear radiology, nucleology), is a medical specialty involving the application of radioactivity, radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, ''radiology done inside out'', because it records radiation radiant exitance, emitted from within the body rather than radiation that is transmittance, transmitted through the body from external sources like X-ray generators. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a Functional imaging, physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine. Diagnostic medical imaging Diagnostic In nuclear medicine imaging, radiopharmaceuticals are taken internally, for example, through inhalation, intravenously, or orally. Then, externa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glenn Seaborg
Glenn Theodore Seaborg ( ; April 19, 1912February 25, 1999) was an American chemist whose involvement in the synthesis, discovery and investigation of ten transuranium elements earned him a share of the 1951 Nobel Prize in Chemistry. His work in this area also led to his development of the actinide concept and the arrangement of the actinide series in the periodic table of the elements. Seaborg spent most of his career as an educator and research scientist at the University of California, Berkeley, serving as a professor, and, between 1958 and 1961, as the university's second chancellor. He advised ten US presidents—from Harry S. Truman to Bill Clinton—on nuclear policy and was Chairman of the United States Atomic Energy Commission from 1961 to 1971, where he pushed for commercial nuclear energy and the peaceful applications of nuclear science. Throughout his career, Seaborg worked for arms control. He was a signatory to the Franck Report and contributed to the Limited T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chernobyl Disaster
On 26 April 1986, the no. 4 reactor of the Chernobyl Nuclear Power Plant, located near Pripyat, Ukrainian Soviet Socialist Republic, Ukrainian SSR, Soviet Union (now Ukraine), exploded. With dozens of direct casualties, it is one of only two nuclear energy accidents rated at the maximum severity on the International Nuclear Event Scale, the other being the 2011 Fukushima nuclear accident. The response involved more than Chernobyl liquidators, 500,000 personnel and cost an estimated 18billion Soviet ruble, rubles (about $84.5billion USD in 2025). It remains the worst nuclear disaster and the List of disasters by cost, most expensive disaster in history, with an estimated cost of US$700 billion. The disaster occurred while running a test to simulate cooling the reactor during an accident in blackout conditions. The operators carried out the test despite an accidental drop in reactor power, and due to a design issue, attempting to shut down the reactor in those conditio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fukushima Daiichi Nuclear Disaster
The Fukushima nuclear accident was a major nuclear accident at the Fukushima Daiichi Nuclear Power Plant in Ōkuma, Fukushima, Japan, which began on 11 March 2011. The cause of the accident was the 2011 Tōhoku earthquake and tsunami, which resulted in electrical grid failure and damaged nearly all of the power plant's Emergency power system, backup energy sources. The subsequent inability to sufficiently cool reactors after shutdown compromised Primary containment, containment and resulted in the release of radioactive contamination, radioactive contaminants into the surrounding environment. The accident was rated seven (the maximum severity) on the International Nuclear Event Scale by Nuclear and Industrial Safety Agency, following a report by the JNES (Japan Nuclear Energy Safety Organization). It is regarded as the worst nuclear incident since the Chernobyl disaster in 1986, which was also rated a seven on the International Nuclear Event Scale. According to the United Nati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally found in its native form as elemental crystals. Tellurium is far more common in the Universe as a whole than on Earth. Its extreme rarity in the Earth's crust, comparable to that of platinum, is due partly to its formation of a volatile hydride that caused tellurium to be lost to space as a gas during the hot nebular formation of Earth. Tellurium-bearing compounds were first discovered in 1782 in a gold mine in Kleinschlatten, Transylvania (now Zlatna, Romania) by Austrian mineralogist Franz-Joseph Müller von Reichenstein, although it was Martin Heinrich Klaproth who named the new element in 1798 after the Latin 'earth'. Gold telluride minerals are the most notable natural gold compounds. However, they are not a commercially signif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |