|
Hofmann Rearrangement
The Hofmann rearrangement (Hofmann degradation) is the organic reaction of a primary amide to a primary amine with one fewer carbon atom. The reaction involves oxidation of the nitrogen followed by rearrangement of the carbonyl and nitrogen to give an isocyanate intermediate. The reaction can form a wide range of products, including alkyl and aryl amines. The reaction is named after its discoverer, August Wilhelm von Hofmann, and should not be confused with the Hofmann elimination, another name reaction for which he is eponymous. Mechanism The reaction of bromine with sodium hydroxide forms sodium hypobromite ''in situ'', which transforms the primary amide into an intermediate isocyanate. The formation of an intermediate nitrene is not possible because it implies also the formation of a hydroxamic acid as a byproduct, which has never been observed. The intermediate isocyanate is hydrolyzed to a primary amine, giving off carbon dioxide. #Base abstracts an acidic N-H proto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
August Wilhelm Von Hofmann
August Wilhelm von Hofmann (8 April 18185 May 1892) was a German chemist who made considerable contributions to organic chemistry. His research on aniline helped lay the basis of the aniline-dye industry, and his research on coal tar laid the groundwork for his student Charles Mansfield's practical methods for extracting benzene and toluene and converting them into nitro compounds and amines. Hofmann's discoveries include formaldehyde, hydrazobenzene, the isonitriles, and allyl alcohol. He prepared three ethylamines and tetraethylammonium compounds and established their structural relationship to ammonia. After studying under Justus von Liebig at the University of Giessen, Hofmann became the first director of the Royal College of Chemistry, now part of Imperial College London, in 1845. In 1865 he returned to Germany to accept a position at the University of Berlin as a teacher and researcher. After his return he co-founded the German Chemical Society (''Deutsche Chemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Situ
''In situ'' (; often not italicized in English) is a Latin phrase that translates literally to "on site" or "in position." It can mean "locally", "on site", "on the premises", or "in place" to describe where an event takes place and is used in many different contexts. For example, in fields such as physics, geology, chemistry, or biology, ''in situ'' may describe the way a measurement is taken, that is, in the same place the phenomenon is occurring without isolating it from other systems or altering the original conditions of the test. The opposite of ''in situ'' is ''ex situ''. Aerospace In the aerospace industry, equipment on-board aircraft must be tested ''in situ'', or in place, to confirm everything functions properly as a system. Individually, each piece may work but interference from nearby equipment may create unanticipated problems. Special test equipment is available for this ''in situ'' testing. It can also refer to repairs made to the aircraft structure or flight con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hoffmann Rearrangement NBS
Hoffmann is a German surname. People A * Albert Hoffmann (1846–1924), German horticulturist * Alexander Hoffmann (born 1975), German politician * Arthur Hoffmann (politician) (1857–1927), Swiss politician and member of the Swiss Federal Council * Asa Hoffmann (born 1943), American chess player *August Heinrich Hoffmann von Fallersleben (1798–1874), German poet B * Banesh Hoffmann (1906–1986), American mathematician and physicist, biographer of Einstein * Baptist Hoffmann (1863–1937), German operatic baritone and voice teacher * Bettina Hoffmann (born 1960), German politician * Bruno Hoffmann (1913–1991), German glass harp player C * Charles F. Hoffmann (1838–1913), German-American topographer * Christoph Hoffmann (1815–1885), German politician and Templer * Christoph Hoffmann (born 1957), German politician D * David Hoffmann (other) E * E. T. A. Hoffmann (Ernst Theodor Amadeus Hoffmann; 1776–1822), German writer, eponym of ''The Tales of Hoffmann'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical and the simplest aliphatic alcohol, with the formula C H3 O H (a methyl group linked to a hydroxyl group, often abbreviated as MeOH). It is a light, volatile, colourless, flammable liquid with a distinctive alcoholic odour similar to that of ethanol (potable alcohol). A polar solvent, methanol acquired the name wood alcohol because it was once produced chiefly by the destructive distillation of wood. Today, methanol is mainly produced industrially by hydrogenation of carbon monoxide. Methanol consists of a methyl group linked to a polar hydroxyl group. With more than 20 million tons produced annually, it is used as a precursor to other commodity chemicals, including formaldehyde, acetic acid, methyl tert-butyl ether, methyl benzoate, anisole, peroxyacids, as well as a host of more specialised chemicals. Occurrence Small amounts of methanol are present in normal, healthy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. History The terms ''nucleophile'' and '' electrophile'' were introduced by Christopher Kelk Ingold in 1933, replacing the terms ''anionoid'' and ''catio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(bis(trifluoroacetoxy)iodo)benzene
(Bis(trifluoroacetoxy)iodo)benzene, , is a hypervalent iodine compound used as a reagent in organic chemistry. It can be used to carry out the Hofmann rearrangement under acidic conditions. Preparation The syntheses of all aryl hypervalent iodine compounds start from iodobenzene. The compound can be prepared by reaction of iodobenzene with a mixture of trifluoroperacetic acid and trifluoroacetic acid in a method analogous to the synthesis of : It can also be prepared by dissolving diacetoxyiodobenzene (a commercially-available compound) with heating in trifluoroacetic acid: : Uses It also brings around the conversion of a hydrazone to a diazo compound, for example in the diazo-thioketone coupling. It also converts thioacetals to their parent carbonyl compounds. Hofmann rearrangement The Hofmann rearrangement is a decarbonylation reaction whereby an amide is converted to an amine by way of an isocyanate intermediate. It is usually carried out under strongly basic conditi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-Bromosuccinimide
''N''-Bromosuccinimide or NBS is a chemical reagent used in radical substitution, electrophilic addition, and electrophilic substitution reactions in organic chemistry. NBS can be a convenient source of Br•, the bromine radical. Preparation NBS is commercially available. It can also be synthesized in the laboratory. To do so, sodium hydroxide and bromine are added to an ice-water solution of succinimide. The NBS product precipitates and can be collected by filtration. Crude NBS gives better yield in the Wohl-Ziegler reaction. In other cases, impure NBS (slightly yellow in color) may give unreliable results. It can be purified by recrystallization from 90 to 95 °C water (10 g of NBS for 100 mL of water). Reactions Addition to alkenes NBS will react with alkenes 1 in aqueous solvents to give bromohydrins 2. The preferred conditions are the portionwise addition of NBS to a solution of the alkene in 50% aqueous DMSO, DME, THF, or ''tert''-butanol at 0&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead(IV) Acetate
Lead(IV) acetate or lead tetraacetate is an organometallic compound with chemical formula . It is a colorless solid that is soluble in nonpolar, organic solvents, indicating that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis. Structure In the solid state the lead(IV) centers are coordinated by four acetate ions, which are bidentate, each coordinating via two oxygen atoms. The lead atom is 8 coordinate and the O atoms form a flattened trigonal dodecahedron. Preparation It is typically prepared by treating of red lead with acetic acid and acetic anhydride (), which absorbs water. The net reaction is shown: :Pb3O4 + 4 Ac2O -> Pb(OAc)4 + 2 Pb(OAc)2 The remaining lead(II) acetate can be partially oxidized to the tetraacetate: :2 Pb(OAc)2 + Cl2 -> Pb(OAc)4 + PbCl2 Reagent in organic chemistry Lead tetraacetate is a strong oxidizing agent, a source of acetyloxy groups and a general reagen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Hypochlorite
Sodium hypochlorite (commonly known in a dilute solution as bleach) is an inorganic chemical compound with the formula NaOCl (or NaClO), comprising a sodium cation () and a hypochlorite anion (or ). It may also be viewed as the sodium salt of hypochlorous acid. The anhydrous compound is unstable and may decompose explosively. It can be crystallized as a pentahydrate ·5, a pale greenish-yellow solid which is not explosive and is stable if kept refrigerated. Sodium hypochlorite is most often encountered as a pale greenish-yellow dilute solution referred to as liquid bleach, which is a household chemical widely used (since the 18th century) as a disinfectant or a bleaching agent. In solution, the compound is unstable and easily decomposes, liberating chlorine, which is the active principle of such products. Sodium hypochlorite is the oldest and still most important chlorine-based bleach. Its corrosive properties, common availability, and reaction products make it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
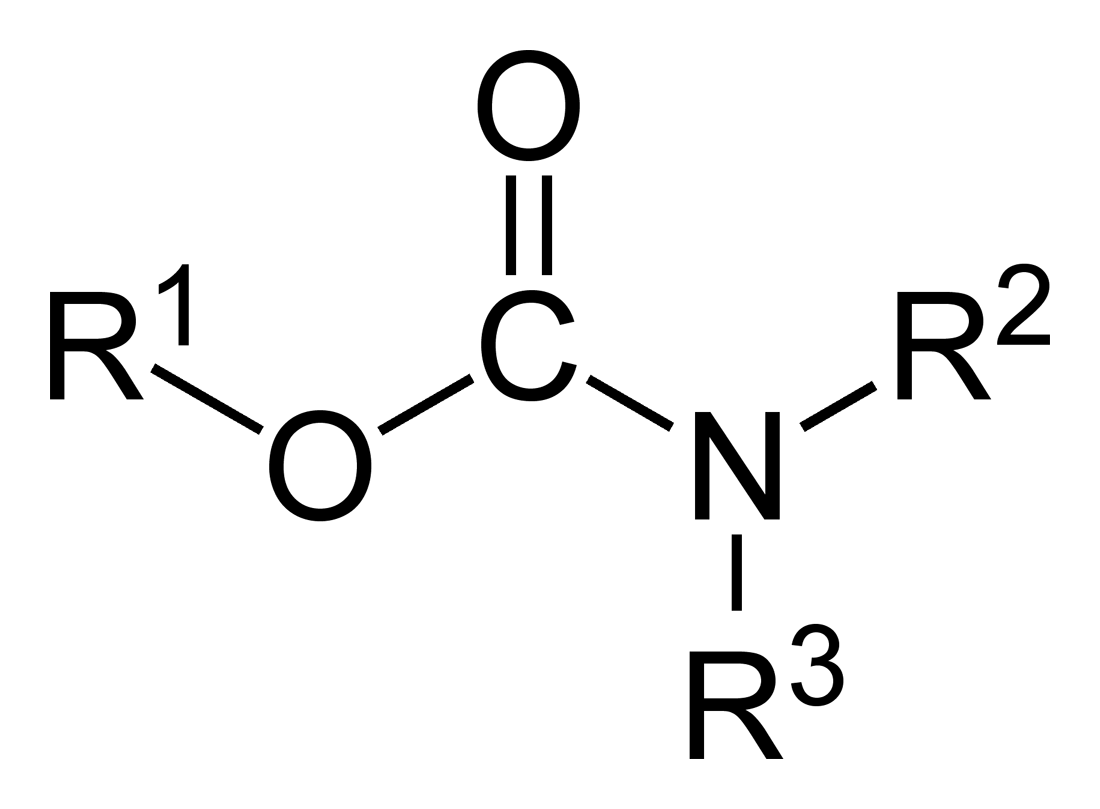
Carbamate
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose units are joined by carbamate groups are an important family of plastics, the polyurethanes. Properties While carbamic acids are unstable, many carbamate esters or ionic) are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate some calcium carbonate imm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbamic Acid
Carbamic acid, which might also be called aminoformic acid or aminocarboxylic acid, is the chemical compound with the formula . It can be obtained by the reaction of ammonia and carbon dioxide at very low temperatures, which also yields an equal amount of ammonium carbamate . The compound is stable only up to about 250 K (−23 °C); at higher temperatures it decomposes into those two gases. The solid apparently consists of dimers, with the two molecules connected by hydrogen bonds between the two carboxyl groups –COOH.J. B. Bossa, P. Theulé, F. Duvernay, F. Borget and T. Chiavassa (2008): "Carbamic acid and carbamate formation in NH3:CO2 ices – UV irradiation versus thermal processes". ''Astronomy and Astrophysics'', volume 492, issue 3, pages 719-724. Carbamic acid could be seen as both an amine and carboxylic acid, and therefore an amino acid;R. K. Khanna and M. H. Moore (1999): "Carbamic acid: molecular structure and IR spectra". ''Spectrochimica Acta Part A: M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hoffman Rearrangement
Hoffman is a surname of German and Jewish origin. The original meaning in medieval times was "steward", i.e. one who manages the property of another. In English and other European languages, including Yiddish and Dutch, the name can also be spelled Hoffmann, Hofmann, Hofman, Huffman, Hofmans. People with the surname A * Aaron Hoffman (1880–1944), American writer, director and comedian * Abbie Hoffman (1936–1989), American social activist of prominence in the 1960s and 1970s * Abraham Hoffman (1938–2015), Israeli basketball player * Al Hoffman (1902–1960), Russian-born American songwriter * Alan Hoffman (born 1982), American entrepreneur * Albert Hofmann (1906–2008), Swiss chemist and discoverer of LSD * Alex Hoffman-Ellis (born 1989), American football player * Alice Hoffman (born 1952), American author * Anthony Hoffman (1739–1790), New York politician * Arthur Sullivant Hoffman (1876–1966), American magazine editor * August Wilhelm von Hofmann (1818-1892) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |