|
HgO
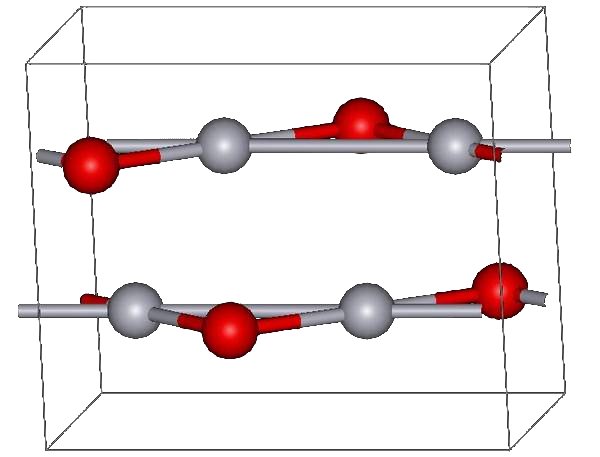
Mercury(II) oxide, also called mercuric oxide or simply mercury oxide, is the inorganic compound with the formula Hg O. It has a red or orange color. Mercury(II) oxide is a solid at room temperature and pressure. The mineral form montroydite is very rarely found. History An experiment for the preparation of mercuric oxide was first described by 11th century Arab-Spanish alchemist, Maslama al-Majriti, in ''Rutbat al-hakim.'' In 1774, Joseph Priestley discovered that oxygen was released by heating mercuric oxide, although he did not identify the gas as oxygen (rather, Priestley called it " dephlogisticated air," as that was the paradigm that he was working under at the time). Synthesis The red form of HgO can be made by heating Hg in oxygen at roughly 350 °C, or by pyrolysis of Hg(NO3)2. The yellow form can be obtained by precipitation of aqueous Hg2+ with alkali. The difference in color is due to particle size; both forms have the same structure consisting of near lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Montroydite
Montroydite is the mineral form of mercury(II) oxide with formula HgO. It is a rare mercury mineral. It was first described for an occurrence in the mercury deposit at Terlingua, Texas and named for Montroyd Sharp who was an owner of the deposit. Montroydite occurs in mercury deposits of hydrothermal origin. Associated minerals include: native mercury, cinnabar, metacinnabar, calomel, eglestonite, terlinguaite, mosesite, kleinite, edgarbaileyite, gypsum Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywal ..., calcite and dolomite. References Mercury(II) minerals Oxide minerals Orthorhombic minerals {{oxide-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Montroydite
Montroydite is the mineral form of mercury(II) oxide with formula HgO. It is a rare mercury mineral. It was first described for an occurrence in the mercury deposit at Terlingua, Texas and named for Montroyd Sharp who was an owner of the deposit. Montroydite occurs in mercury deposits of hydrothermal origin. Associated minerals include: native mercury, cinnabar, metacinnabar, calomel, eglestonite, terlinguaite, mosesite, kleinite, edgarbaileyite, gypsum Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula . It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard or sidewalk chalk, and drywal ..., calcite and dolomite. References Mercury(II) minerals Oxide minerals Orthorhombic minerals {{oxide-mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnabar
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury and is the historic source for the brilliant red or scarlet pigment termed vermilion and associated red mercury pigments. Cinnabar generally occurs as a vein-filling mineral associated with recent volcanic activity and alkaline hot springs. The mineral resembles quartz in symmetry and in its exhibiting birefringence. Cinnabar has a mean refractive index near 3.2, a hardness between 2.0 and 2.5, and a specific gravity of approximately 8.1. The color and properties derive from a structure that is a hexagonal crystalline lattice belonging to the trigonal crystal system, crystals that sometimes exhibit twinning. Cinnabar has been used for its color since antiquity in the Near East, including as a rouge-type cosmetic, in the New World since the Olmec culture, and in C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnabar Structure
Cinnabar (), or cinnabarite (), from the grc, κιννάβαρι (), is the bright scarlet to brick-red form of mercury(II) sulfide (HgS). It is the most common source ore for refining elemental mercury and is the historic source for the brilliant red or scarlet pigment termed vermilion and associated red mercury pigments. Cinnabar generally occurs as a vein-filling mineral associated with recent volcanic activity and alkaline hot springs. The mineral resembles quartz in symmetry and in its exhibiting birefringence. Cinnabar has a mean refractive index near 3.2, a hardness between 2.0 and 2.5, and a specific gravity of approximately 8.1. The color and properties derive from a structure that is a hexagonal crystalline lattice belonging to the trigonal crystal system, crystals that sometimes exhibit twinning. Cinnabar has been used for its color since antiquity in the Near East, including as a rouge-type cosmetic, in the New World since the Olmec culture, and in China since ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maslama Al-Majriti
Abu al-Qasim Maslama ibn Ahmad al-Majriti ( ar, أبو القاسم مسلمة بن أحمد المجريطي: c. 950–1007), known or Latin as , was an Arab Muslim astronomer, chemist, mathematician, economist and Scholar in Islamic Spain, active during the reign of Al-Hakam II. His full name is Abu ’l-Qāsim Maslama ibn Aḥmad al-Faraḍī al-Ḥāsib al-Maj̲rīṭī al-Qurṭubī al-Andalusī. Achievements Al-Majrīṭī took part in the translation of Ptolemy's '' Planisphaerium'', improved existing translations of the '' Almagest'', introduced and improved the astronomical tables of Muhammad ibn Musa al-Khwarizmi, aided historians by working out tables to convert Persian dates to Hijri years, and introduced the techniques of surveying and triangulation. According to Said al-Andalusi, he was the best mathematician and astronomer of his time in al-Andalus. He also introduced new surveying methods by working closely with his colleague ibn al-Saffar. He also wrote a b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as well as with other compounds. Oxygen is Earth's most abundant element, and after hydrogen and helium, it is the third-most abundant element in the universe. At standard temperature and pressure, two atoms of the element bind to form dioxygen, a colorless and odorless diatomic gas with the formula . Diatomic oxygen gas currently constitutes 20.95% of the Earth's atmosphere, though this has changed considerably over long periods of time. Oxygen makes up almost half of the Earth's crust in the form of oxides.Atkins, P.; Jones, L.; Laverman, L. (2016).''Chemical Principles'', 7th edition. Freeman. Many major classes of organic molecules in living organisms contain oxygen atoms, such as proteins, nucleic acids, carbohydrates, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury (element)
Mercury is a chemical element with the symbol Hg and atomic number 80. It is also known as quicksilver and was formerly named hydrargyrum ( ) from the Greek words, ''hydor'' (water) and ''argyros'' (silver). A heavy, silvery d-block element, mercury is the only metallic element that is known to be liquid at standard temperature and pressure; the only other element that is liquid under these conditions is the halogen bromine, though metals such as caesium, gallium, and rubidium melt just above room temperature. Mercury occurs in deposits throughout the world mostly as cinnabar ( mercuric sulfide). The red pigment vermilion is obtained by grinding natural cinnabar or synthetic mercuric sulfide. Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps and other devices, though concerns about the element's toxicity have led to mercury thermometers and sphygmomanometers being largely p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetragonal
In crystallography, the tetragonal crystal system is one of the 7 crystal systems. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (''a'' by ''a'') and height (''c'', which is different from ''a''). Bravais lattices There are two tetragonal Bravais lattices: the primitive tetragonal and the body-centered tetragonal. The base-centered tetragonal lattice is equivalent to the primitive tetragonal lattice with a smaller unit cell, while the face-centered tetragonal lattice is equivalent to the body-centered tetragonal lattice with a smaller unit cell. Crystal classes The point groups that fall under this crystal system are listed below, followed by their representations in international notation, Schoenflies notation, orbifold notation, Coxeter notation and mineral examples.Hurlbut, Cornelius S.; Klein, Cornelis, 1985, ''Manual of Mineralogy'', 20th ed., ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexagonal Crystal System
In crystallography, the hexagonal crystal family is one of the six crystal families, which includes two crystal systems (hexagonal and trigonal) and two lattice systems (hexagonal and rhombohedral). While commonly confused, the trigonal crystal system and the rhombohedral lattice system are not equivalent (see section crystal systems below). In particular, there are crystals that have trigonal symmetry but belong to the hexagonal lattice (such as α-quartz). The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. There are 52 space groups associated with it, which are exactly those whose Bravais lattice is either hexagonal or rhombohedral. __TOC__ Lattice systems The hexagonal crystal family consists of two lattice systems: hexagonal and rhombohedral. Each lattice system consists of one Bravai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the prim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Nitrate
Mercury(II) nitrate is an inorganic compound with the formula Hg(NO3)2.xH2O. These colorless or white soluble crystalline salts are occasionally used as a reagent. It is made by treating mercury with hot concentrated nitric acid. Neither anhydrous nor monohydrate has been confirmed by X-ray crystallography. The anhydrous material is more widely used. Uses Mercuric nitrate has been used in mercuration of ketones. Mercuric nitrate was formerly used in carroting felt for hats. Health information Mercury compounds are highly toxic. The use of this compound by hatters and the subsequent mercury poisoning of said hatters is a common theory of where the phrase " mad as a hatter" came from. See also * The Hatter The Hatter is a fictional character in Lewis Carroll's 1865 book ''Alice's Adventures in Wonderland'' and its 1871 sequel ''Through the Looking-Glass''. He is very often referred to as the Mad Hatter, though this term was never used by Ca ... * Mercury pois ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyro'' "fire", "heat", "fever" and '' lysis'' "separating". Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.''Burning of wood'' , InnoFireWood's website. Accessed on 2010-02-06. In general, pyrolysis of organic substances produces volatile products and leaves char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly |