|
Henderson–Hasselbalch Equation
In chemistry and biochemistry, the Henderson–Hasselbalch equation :\ce = \ceK_\ce + \log_ \left( \frac \right) relates the pH of a chemical solution of a weak acid to the numerical value of the acid dissociation constant, ''K''a, of acid and the ratio of the concentrations, \frac of the acid and its conjugate base in an equilibrium. : \mathrm For example, the acid may be acetic acid :\mathrm The Henderson–Hasselbalch equation can be used to estimate the pH of a buffer solution by approximating the actual concentration ratio as the ratio of the analytical concentrations of the acid and of a salt, MA. The equation can also be applied to bases by specifying the protonated form of the base as the acid. For example, with an amine, \mathrm :\mathrm Derivation, assumptions and limitations A simple buffer solution consists of a solution of an acid and a salt of the conjugate base of the acid. For example, the acid may be acetic acid and the salt may be sodium acetate. The Hend ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a natural science that covers the elements that make up matter to the compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during a reaction with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both basic and applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth ( botany), the formation of igneous rocks ( geology), how atmospheric ozone is formed and how environmental pollutants are degraded ( ecology), the properties of the soil on the moon ( cosmochemistry), how medications work ( pharmacology), and how to collec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Sulphate
Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, soluble in water but not in ethanol. Magnesium sulfate is usually encountered in the form of a hydrate , for various values of ''n'' between 1 and 11. The most common is the heptahydrate , known as Epsom salt, which is a household chemical with many traditional uses, including bath salts. The main use of magnesium sulfate is in agriculture, to correct soils deficient in magnesium (an essential plant nutrient because of the role of magnesium in chlorophyll and photosynthesis). The monohydrate is favored for this use; by the mid 1970s, its production was 2.3 million tons per year. The anhydrous form and several hydrates occur in nature as minerals, and the salt is a significant component of the water from some springs. Hydrates Magnesium s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biochemische Zeitschrift
''The FEBS Journal'' is a biweekly peer-reviewed scientific journal published by John Wiley & Sons on behalf of the Federation of European Biochemical Societies. It covers research on all aspects of biochemistry, molecular biology, cell biology, and the molecular bases of disease. The editor-in-chief is Seamus Martin (Trinity College Dublin), who took over from Richard Perham (University of Cambridge) in 2014. Content is available for free 1 year after publication, except review content, which is available immediately. The journal also publishes special and virtual issues focusing on a specific theme. Since 2021, the journal has given an annual award, "The FEBS Journal Richard Perham Prize", for an outstanding research paper published in the journal. The winners receive a €5,000 cash prize (to be divided equally between the first and last authors) and the senior author of the study is invited to give a talk at the FEBS Annual Congress. The journal also gives more frequent post ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Logarithm
In mathematics, the logarithm is the inverse function to exponentiation. That means the logarithm of a number to the base is the exponent to which must be raised, to produce . For example, since , the ''logarithm base'' 10 of is , or . The logarithm of to ''base'' is denoted as , or without parentheses, , or even without the explicit base, , when no confusion is possible, or when the base does not matter such as in big O notation. The logarithm base is called the decimal or common logarithm and is commonly used in science and engineering. The natural logarithm has the number e (mathematical constant), as its base; its use is widespread in mathematics and physics, because of its very simple derivative. The binary logarithm uses base and is frequently used in computer science. Logarithms were introduced by John Napier in 1614 as a means of simplifying calculations. They were rapidly adopted by navigators, scientists, engineers, surveyors and oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Karl Albert Hasselbalch
Karl Albert Hasselbalch (; 1 November 1874 – 19 September 1962) was a Danish physician and chemist known for his work on the Henderson–Hasselbalch equation. Early life and education Hasselbalch was born in Åstrup, near Hjørring, Denmark on 1 November 1874. Hasselbalch received his medical degree in 1898 and his doctorate in 1899 for his thesis on the respiratory metabolism in the chicken embryo. Career Hasselbalch was a pioneer in the use of pH measurement in medicine (with Christian Bohr, father of Niels Bohr), and he described how the affinity of blood for oxygen was dependent on the concentration of carbon dioxide. He was also first to determine the pH of blood. In 1916, he converted the 1908 equation of Lawrence Joseph Henderson to logarithmic form, which is now known as the Henderson–Hasselbalch equation In chemistry and biochemistry, the Henderson–Hasselbalch equation :\ce = \ceK_\ce + \log_ \left( \frac \right) relates the pH of a chemical solution of a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Søren Peter Lauritz Sørensen
Søren (, ) or Sören (, ) is a Scandinavian given name that is sometimes Anglicized as Soren. The name is derived from that of the 4th-century Christian saint Severin of Cologne,Portal Rheinische Geschichte"Severin (circa 330-400), Heiliger und Bischof von Köln (397)"/ref> ultimately derived from the Latin ''severus'' ("severe, strict, serious"). Its feminine form is Sørine, though its use is uncommon. The patronymic surname Sørensen is derived from Søren. List of people with the given name Søren *Soren Sorensen Adams (1879–1963), American inventor * Søren Berg (born 1976), Danish football player * Søren "Bjergsen" Bjerg (born 1996), Danish ''League of Legends'' player * Søren Brorsen (1875–1961), Danish politician *Søren Gade (born 1963), Danish politician * Sören Johansson (born 1954), Swedish ice hockey player * Soren Johnson, American game designer * Søren Kierkegaard (1813–1855), Danish philosopher *Søren Larsen (born 1981), Danish football player *Søre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicarbonate Buffer System
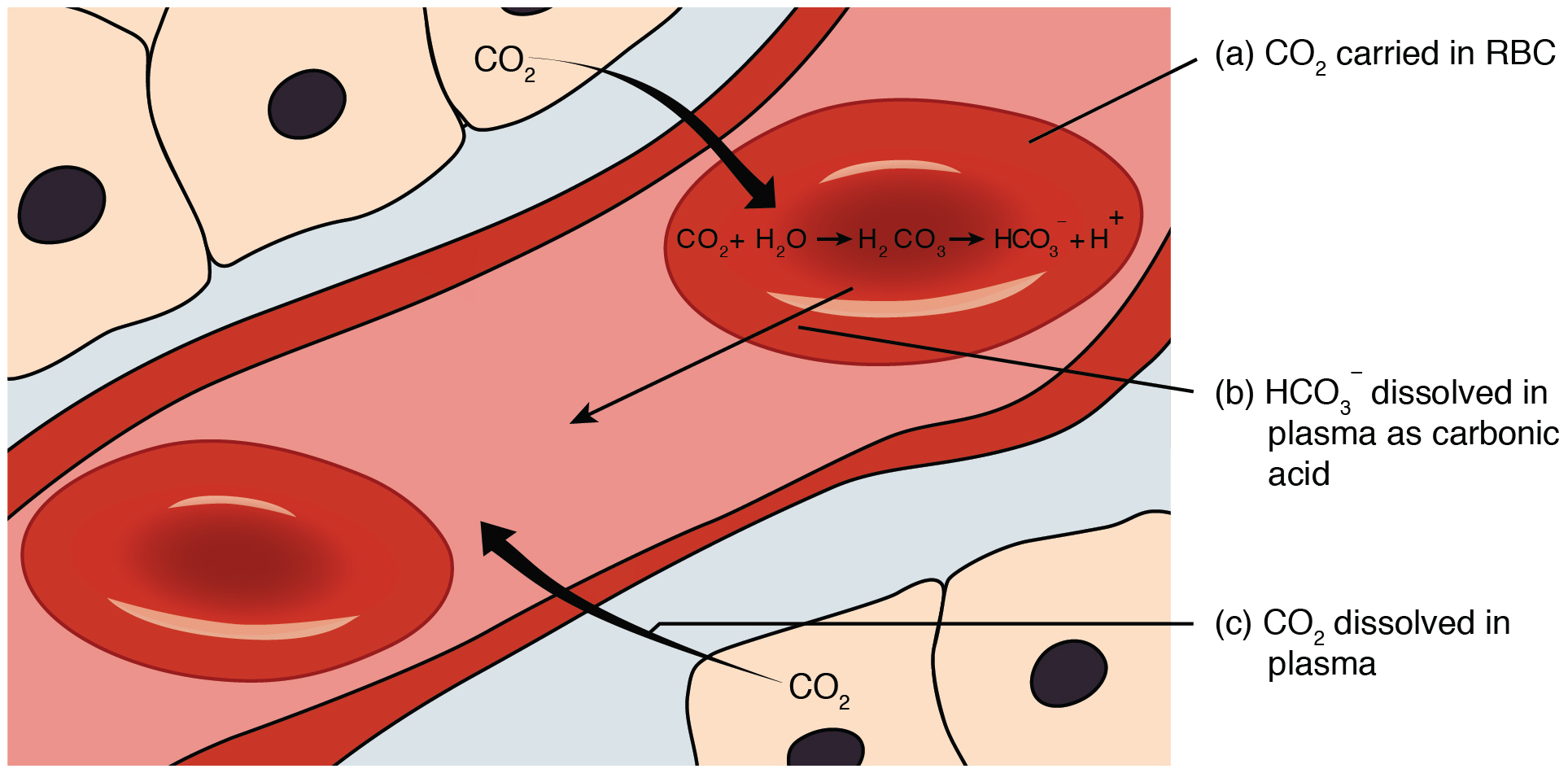
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO), and carbon dioxide (CO2) in order to maintain pH in the blood and duodenum, among other tissues, to support proper metabolic function. Catalyzed by carbonic anhydrase, carbon dioxide (CO2) reacts with water (H2O) to form carbonic acid (H2CO3), which in turn rapidly dissociates to form a bicarbonate ion (HCO ) and a hydrogen ion (H+) as shown in the following reaction: \rm CO_2 + H_2O \rightleftarrows H_2CO_3 \rightleftarrows HCO_3^- + H^+ As with any buffer system, the pH is balanced by the presence of both a weak acid (for example, H2CO3) and its conjugate base (for example, HCO) so that any excess acid or base introduced to the system is neutralized. Failure of this system to function properly results in acid-base imbalance, such as acidemia (pH 7.45) in the blood. In systemic acid–base balance In tissue, cellular respiration produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lawrence Joseph Henderson
Lawrence Joseph Henderson (June 3, 1878, Lynn, Massachusetts – February 10, 1942, Cambridge, Massachusetts) was a physiologist, chemist, biologist, philosopher, and sociologist. He became one of the leading biochemists of the early 20th century. His work contributed to the Henderson–Hasselbalch equation, used to calculate pH as a measure of acidity. Early life Lawrence Henderson was born in Lynn, Massachusetts the son of a business man Joseph Henderson and his wife. He entered Harvard at the age of 16 in 1894. His father was a ship chandler whose principal business was located in nearby Salem, but who also conducted business in Saint Pierre and Miquelon, a French Overseas collectivity off the coast of Canada. Career Lawrence Henderson graduated from Harvard College in 1898 and from Harvard Medical School in 1902, receiving the M.D. (Medical Doctor) degree cum laude. Then followed two years in chemical research at the University of Strasbourg with advanced scienti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Partial Pressure
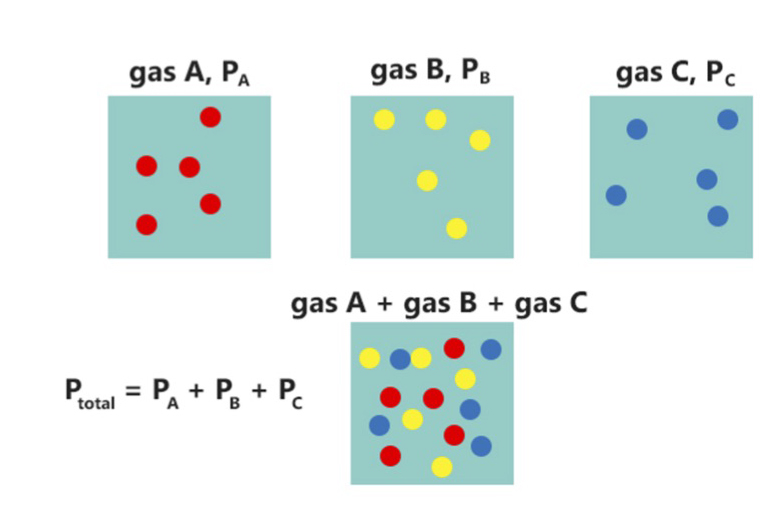
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture ( Dalton's Law). The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula . Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid . The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid ; and the conjugate acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |