|
H₂IMes
SIMes (or H2Imes) is an ''N''-heterocyclic carbene. It is a white solid that dissolves in organic solvents. The compound is used as a ligand in organometallic chemistry. It is structurally related to the more common ligand IMes IMes is an abbreviation for an organic compound that is a common ligand in organometallic chemistry. It is an ''N''-heterocyclic carbene (NHC). The compound, a white solid, is often not isolated but instead is generated upon attachment to the me ... but with a saturated backbone (the S of SIMes indicates a saturated backbone). It is slightly more flexible and is a component in Grubbs II. It is prepared by alkylation of trimethylaniline by dibromoethane followed by ring closure and dehydrohalogenation. References {{organic-compound-stub Carbenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grubbs' Catalyst
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have also been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in Organic Chemistry#Organic synthesis, synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis. First-generation Grubbs catalyst In the 1960s, ruthenium trichloride was found to catalyze olefin metathesis. Processes were commercialized based on these discoveries. These ill-defined but highly active homogeneous catalysts remain in industrial use. The first well-defined ruthenium catalyst was rep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
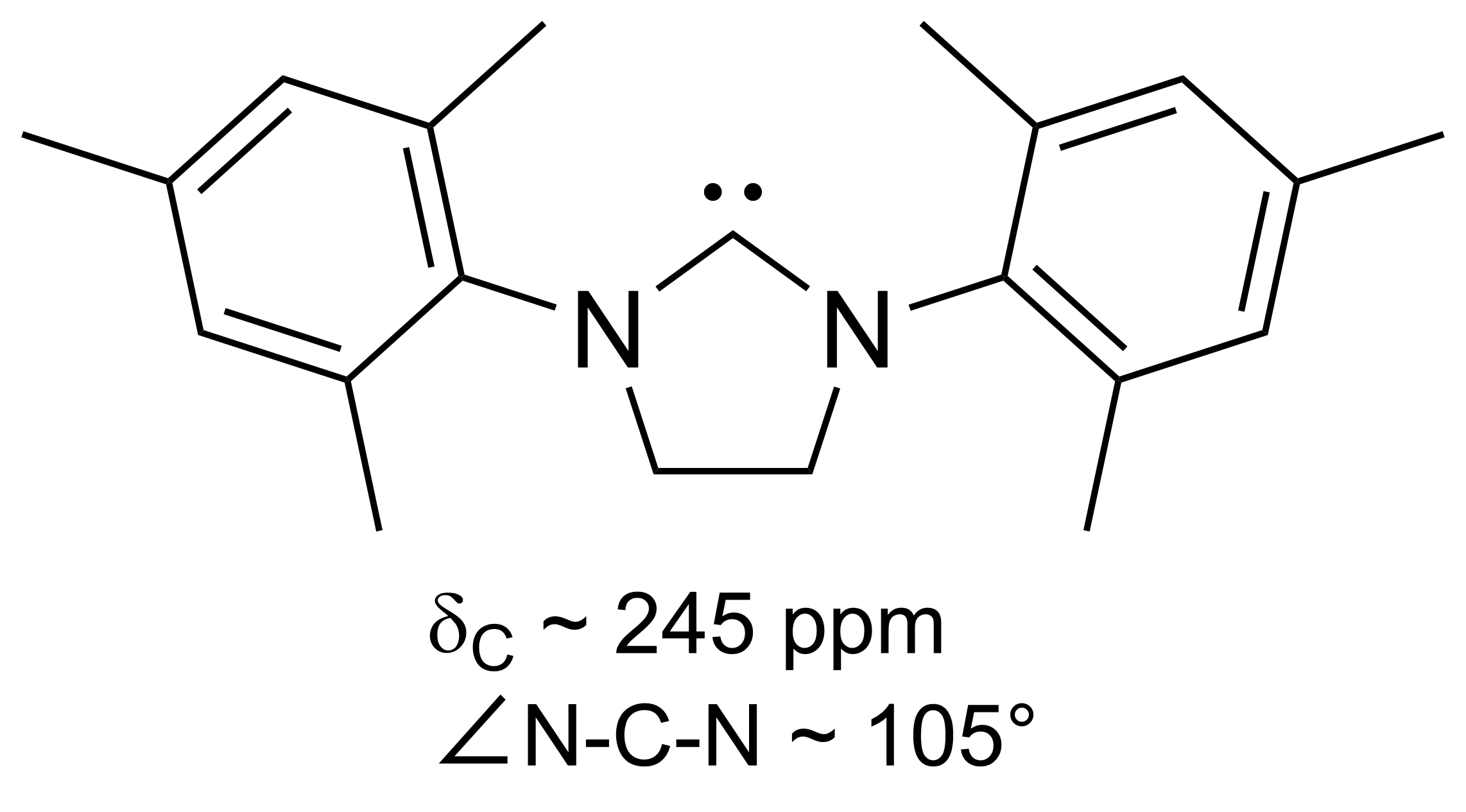
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with octet rule, incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), in which nitrogen atoms flank the formal carbene. Modern theoretical analysis suggests that the term "persistent carbene" is in fact a misnomer. Persistent carbenes do not in fact have a carbene electronic structure in their ground state, but instead an ylide stabilized by Aromaticity, aromatic resonance or steric shielding. Excitation to a carbene structure then accounts for the carbene-like dimerization that some persistent carbenes undergo over the course of days. Persistent carbenes in general, and Arduengo carbenes in particular, are popular ligands in organometallic chemistry. Histor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (Metal carbonyl, metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganics, metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IMes
IMes is an abbreviation for an organic compound that is a common ligand in organometallic chemistry. It is an ''N''-heterocyclic carbene (NHC). The compound, a white solid, is often not isolated but instead is generated upon attachment to the metal centre. First prepared by Arduengo, the heterocycle is synthesized by condensation of 2,4,6-trimethylaniline and glyoxal to give the diimine. In the presence of acid, the resulting glyoxal-bis(mesitylimine) condenses with formaldehyde to give the dimesityl imidazolium cation. This cation is the conjugate acid of the NHC. Related compounds Bulkier than IMes is the NHC ligand IPr ( CAS 244187-81-3). IPr features diisopropylphenyl in place of the mesityl substituents. Some variants of IMes and IPr have saturated backbones, two such ligands are SIMes SIMes (or H2Imes) is an N-heterocyclic carbene, ''N''-heterocyclic carbene. It is a white solid that dissolves in organic solvents. The compound is used as a ligand in organome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |