|
Helium-3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron. (In contrast, the most common isotope, helium-4, has two protons and two neutrons.) Helium-3 and hydrogen-1 are the only stable nuclides with more protons than neutrons. It was discovered in 1939. Helium-3 atoms are fermionic and become a superfluid at the temperature of 2.491 mK. Helium-3 occurs as a primordial nuclide, escaping from Earth's crust into its atmosphere and into outer space over millions of years. It is also thought to be a natural nucleogenic and cosmogenic nuclide, one produced when lithium is bombarded by natural neutrons, which can be released by spontaneous fission and by nuclear reactions with cosmic rays. Some found in the terrestrial atmosphere is a remnant of atmospheric and underwater nuclear weapons testing. Nuclear fusion using helium-3 has long been viewed as a desirable future energy source. The fusion of two of its atoms would be aneut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aneutronic Fusion
Aneutronic fusion is any form of fusion power in which very little of the energy released is carried by Neutron, neutrons. While the lowest-threshold Nuclear fusion#Important reactions, nuclear fusion reactions release up to 80% of their energy in the form of neutrons, aneutronic reactions release energy in the form of Charged particle, charged particles, typically protons or alpha particles. Successful aneutronic fusion would greatly reduce problems associated with neutron radiation such as damaging ionizing radiation, neutron activation, reactor maintenance, and requirements for biological shielding, remote handling and safety. Since it is simpler to convert the energy of charged particles into electrical power than it is to convert energy from uncharged particles, an aneutronic reaction would be attractive for power systems. Some proponents see a potential for dramatic cost reductions by converting energy directly to electricity, as well as in eliminating the radiation from neut ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the Chemical element, elements, and it does not have a melting point at standard pressures. It is the second-lightest and second-most Abundance of the chemical elements, abundant element in the observable universe, after hydrogen. It is present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined. Its abundance is similar to this in both the Sun and Jupiter, because of the very high nuclear binding energy (per nucleon) of helium-4 with respect to the next three elements after helium. This helium-4 binding energy also accounts for why it is a product of both nuclear fusion and radioactive decay. The most common isotope of helium in the universe is helium-4, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helion (chemistry)
A helion (symbol h) is the nucleus of a helium atom, a doubly positively charged cation. The term ''helion'' is a portmanteau of ''helium'' and ''ion'', and in practice refers specifically to the nucleus of the helium-3 isotope, consisting of two protons and one neutron. The nucleus of the other (and far more common) stable isotope of helium, helium-4, consisting of two protons and two neutrons, is called an '' alpha particle'' or an alpha for short. This particle is the daughter product in the beta-minus decay of tritium, an isotope of hydrogen: : CODATA reports the mass of a helion particle as = Helions are intermediate products in the proton–proton chain reaction in stellar fusion. An antihelion is the antiparticle In particle physics, every type of particle of "ordinary" matter (as opposed to antimatter) is associated with an antiparticle with the same mass but with opposite physical charges (such as electric charge). For example, the antiparticle of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cosmic Ray
Cosmic rays or astroparticles are high-energy particles or clusters of particles (primarily represented by protons or atomic nuclei) that move through space at nearly the speed of light. They originate from the Sun, from outside of the Solar System in our own galaxy, and from distant galaxies. Upon impact with Earth's atmosphere, cosmic rays produce showers of secondary particles, some of which reach the surface, although the bulk are deflected off into space by the magnetosphere or the heliosphere. Cosmic rays were discovered by Victor Hess in 1912 in balloon experiments, for which he was awarded the 1936 Nobel Prize in Physics. Direct measurement of cosmic rays, especially at lower energies, has been possible since the launch of the first satellites in the late 1950s. Particle detectors similar to those used in nuclear and high-energy physics are used on satellites and space probes for research into cosmic rays. Data from the Fermi Space Telescope (2013) have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotopes Of Hydrogen
Hydrogen (H) has three naturally occurring isotopes: H, H, and H. H and H are stable, while H has a half-life of years. Heavier isotopes also exist; all are synthetic and have a half-life of less than 1 zeptosecond (10 s). Of these, H is the least stable, while H is the most. Hydrogen is the only element whose isotopes have different names that remain in common use today: H is deuterium and H is tritium. The symbols D and T are sometimes used for deuterium and tritium; IUPAC (International Union of Pure and Applied Chemistry) accepts said symbols, but recommends the standard isotopic symbols H and H, to avoid confusion in alphabetic sorting of chemical formulas. H, with no neutrons, may be called protium to disambiguate. (During the early study of radioactivity, some other heavy radioisotopes were given names, but such names are rarely used today.) List of isotopes Note: "y" means year, but "ys" means yoctosecond (10 second). , - , H , 1 , 0 , , colspan=3 al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stable Isotope
Stable nuclides are Isotope, isotopes of a chemical element whose Nucleon, nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The Atomic nucleus, nuclei of such isotopes are not radioactive and unlike radionuclides do not spontaneously undergo radioactive decay. When these nuclides are referred to in relation to specific elements they are usually called that element's stable isotopes. The 80 elements with one or more stable isotopes comprise a total of 251 nuclides that have not been shown to decay using current equipment. Of these 80 elements, 26 have only one stable isotope and are called monoisotopic element, monoisotopic. The other 56 have more than one stable isotope. Tin has ten stable isotopes, the largest number of any element. Definition of stability, and naturally occurring nuclides Most naturally occurring nuclides are stable (about 251; see list at the end of this article), and about 35 more (tot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Giant
A gas giant is a giant planet composed mainly of hydrogen and helium. Jupiter and Saturn are the gas giants of the Solar System. The term "gas giant" was originally synonymous with "giant planet". However, in the 1990s, it became known that Uranus and Neptune are a distinct class of giant planets composed mainly of heavier volatile substances (referred to as "Volatile (astrogeology)#Planetary science, ices"). For this reason, Uranus and Neptune are often classified in the separate category of ice giants. Jupiter and Saturn consist mostly of hydrogen and helium, with heavier elements making up between 3 and 13 percent of their mass.The Interior of Jupiter, Guillot et al., in ''Jupiter: The Planet, Satellites and Magnetosphere'', Bagenal et al., editors, Cambridge University Press, 2004 They are thought to have an outer layer of compressed molecular hydrogen surrounding a layer of liquid metallic hydrogen, with a molten rocky core inside. The outermost portion of their hydrogen atmo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
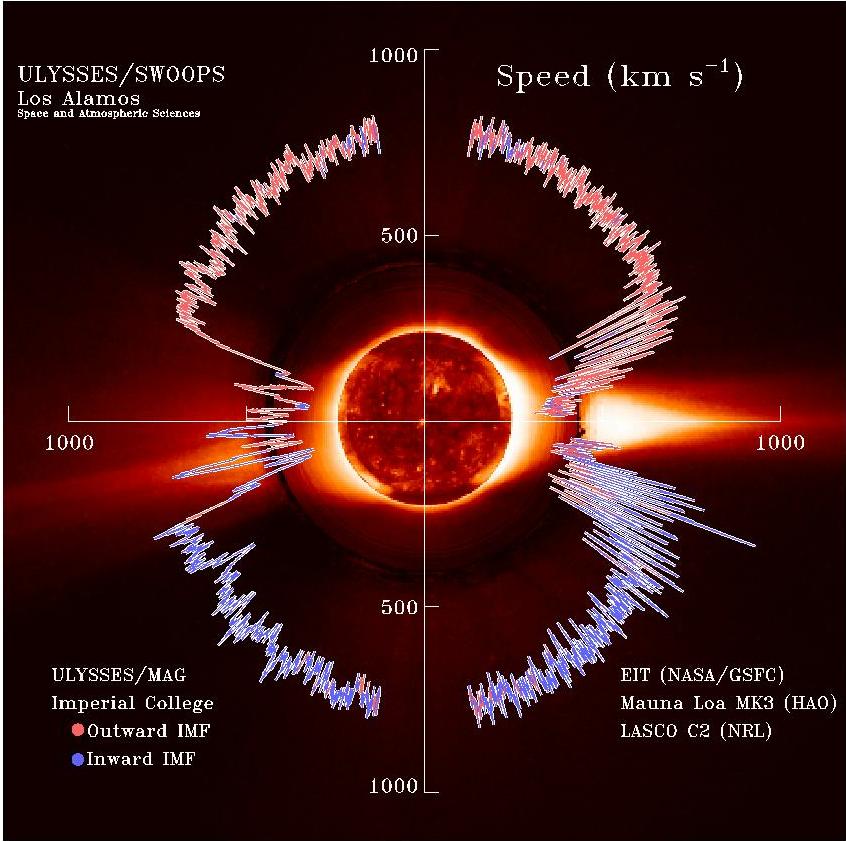
Solar Wind
The solar wind is a stream of charged particles released from the Sun's outermost atmospheric layer, the Stellar corona, corona. This Plasma (physics), plasma mostly consists of electrons, protons and alpha particles with kinetic energy between . The composition of the solar wind plasma also includes a mixture of particle species found in the solar plasma: trace amounts of heavy ions and atomic nuclei of Chemical element, elements such as carbon, nitrogen, oxygen, neon, magnesium, silicon, sulfur, and iron. There are also rarer traces of some other nuclei and isotopes such as phosphorus, titanium, chromium, and nickel's isotopes 58Ni, 60Ni, and 62Ni. Superimposed with the solar-wind plasma is the interplanetary magnetic field. The solar wind varies in density, temperature and speed over time and over Solar coordinate systems#Heliographic, solar latitude and longitude. Its particles can escape the Sun's gravity because of their high energy resulting from the high temperature of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Regolith
Regolith () is a blanket of unconsolidated, loose, heterogeneous superficial deposits covering solid rock. It includes dust, broken rocks, and other related materials and is present on Earth, the Moon, Mars, some asteroids, and other terrestrial planets and moons. Etymology The term '' regolith'' combines two Greek words: (), 'blanket', and (), 'rock'. The American geologist George P. Merrill first defined the term in 1897, writing: Earth Earth's regolith includes the following subdivisions and components: * soil or pedolith * alluvium and other transported cover, including that transported by aeolian, glacial, marine, and gravity flow processes. * "saprolith'", generally divided into the ** ''upper saprolite'': completely oxidised bedrock ** ''lower saprolite'': chemically reduced partially weathered rocks ** ''saprock'': fractured bedrock with weathering restricted to fracture margins * volcanic ash and lava flows that are interbedded with unconsolidated materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spontaneous Fission
Spontaneous fission (SF) is a form of radioactive decay in which a heavy atomic nucleus splits into two or more lighter nuclei. In contrast to induced fission, there is no inciting particle to trigger the decay; it is a purely probabilistic process. Spontaneous fission is a dominant decay mode for superheavy elements, with nuclear stability generally falling as nuclear mass increases. It thus forms a practical limit to heavy element nucleon number. Heavier nuclides may be created instantaneously by physical processes, both natural (via the r-process, ''r''-process) and artificial, though rapidly decay to more stable nuclides. As such, apart from minor decay branches in primordial radionuclides, spontaneous fission is not observed in nature. Observed fission half-lives range from 60 nanoseconds () to greater than the current age of the universe (). History Following the discovery of induced fission by Otto Hahn and Fritz Strassmann in 1938, Soviet physicists Georgy Flyorov and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter. Nuclear physics should not be confused with atomic physics, which studies the atom as a whole, including its electrons. Discoveries in nuclear physics have led to applications in many fields such as nuclear power, nuclear weapons, nuclear medicine and magnetic resonance imaging, industrial and agricultural isotopes, ion implantation in materials engineering, and radiocarbon dating in geology and archaeology. Such applications are studied in the field of nuclear engineering. Particle physics evolved out of nuclear physics and the two fields are typically taught in close association. Nuclear astrophysics, the application of nuclear physics to astrophysics, is crucial in explaining the inner workings of stars and the origin of the chemical elements. History The history of nuclear physics as a discipline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other by the number of protons that are in their atoms. For example, any atom that contains 11 protons is sodium, and any atom that contains 29 protons is copper. Atoms with the same number of protons but a different number of neutrons are called isotopes of the same element. Atoms are extremely small, typically around 100 picometers across. A human hair is about a million carbon atoms wide. Atoms are smaller than the shortest wavelength of visible light, which means humans cannot see atoms with conventional microscopes. They are so small that accurately predicting their behavior using classical physics is not possible due to quantum mechanics, quantum effects. More than 99.94% of an atom's mass is in the nucleus. Protons hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |