|
Graham Condenser
In chemistry, a condenser is laboratory apparatus used to condense vaporsthat is, turn them into liquidsby cooling them down. Condensers are routinely used in laboratory operations such as distillation, reflux, and extraction. In distillation, a mixture is heated until the more volatile components boil off, the vapors are condensed, and collected in a separate container. In reflux, a reaction involving volatile liquids is carried out at their boiling point, to speed it up; and the vapors that inevitably come off are condensed and returned to the reaction vessel. In Soxhlet extraction, a hot solvent is infused onto some powdered material, such as ground seeds, to leach out some poorly soluble component; the solvent is then automatically distilled out of the resulting solution, condensed, and infused again. Many different types of condensers have been developed for different applications and processing volumes. The simplest and oldest condenser is just a long tube through wh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distillation 2-3
Distillation, or classical distillation, is the process of separating the components or substances from a liquid mixture by using selective boiling and condensation, usually inside an apparatus known as a still. Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids); this may involve chemical changes such as destructive distillation or cracking. Distillation may result in essentially complete separation (resulting in nearly pure components), or it may be a partial separation that increases the concentration of selected components; in either case, the process exploits differences in the relative volatility of the mixture's components. In industrial applications, distillation is a unit operation of practically universal importance, but is a physical separation process, not a chemical reaction. An installation used for distillation, especially of distilled beverages, is a distillery. Distillation includes the fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Johan Gadolin
Johan Gadolin (5 June 176015 August 1852) was a Finnish chemist, physicist and mineralogist. Gadolin discovered a " new earth" containing the first rare-earth compound yttrium, which was later determined to be a chemical element. He is also considered the founder of Finnish chemistry research, as the second holder of the Chair of Chemistry at the Royal Academy of Turku (or ''Åbo Kungliga Akademi''). Gadolin was ennobled for his achievements and awarded the Order of Saint Vladimir and the Order of Saint Anna. Early life and education Johan Gadolin was born in Åbo (Finnish name Turku), Finland (then a part of Sweden). Johan was the son of Jakob Gadolin, professor of physics and theology at Åbo. Johan began to study mathematics at the Royal Academy of Turku (''Åbo Kungliga Akademi'') when he was fifteen. Later he changed his major to chemistry, studying with Pehr Adrian Gadd, the first chair of chemistry at Åbo. In 1779 Gadolin moved to Uppsala University. In 1781, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kilopascal
The pascal (symbol: Pa) is the unit of pressure in the International System of Units (SI), and is also used to quantify internal pressure, stress, Young's modulus, and ultimate tensile strength. The unit, named after Blaise Pascal, is defined as one newton per square metre and is equivalent to 10 barye (Ba) in the CGS system. The unit of measurement called standard atmosphere (atm) is defined as 101,325 Pa. Common multiple units of the pascal are the hectopascal (1 hPa = 100 Pa), which is equal to one millibar, and the kilopascal (1 kPa = 1000 Pa), which is equal to one centibar. Meteorological observations typically report atmospheric pressure in hectopascals per the recommendation of the World Meteorological Organization, thus a standard atmosphere (atm) or typical sea-level air pressure is about 1013 hPa. Reports in the United States typically use inches of mercury or millibars (hectopascals). In Canada these reports are given in kilopascal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is transparent to visible light but absorbs infrared radiation, acting as a greenhouse gas. It is a trace gas in Earth's atmosphere at 421 parts per million (ppm), or about 0.04% by volume (as of May 2022), having risen from pre-industrial levels of 280 ppm. Burning fossil fuels is the primary cause of these increased CO2 concentrations and also the primary cause of climate change.IPCC (2022Summary for policy makersiClimate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA Carbon dioxide is soluble in water and is found in groundwater, lakes, ice ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was , coming mostly from Brazil and the U.S. Etymology ''Ethanol'' is the systematic name defined by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
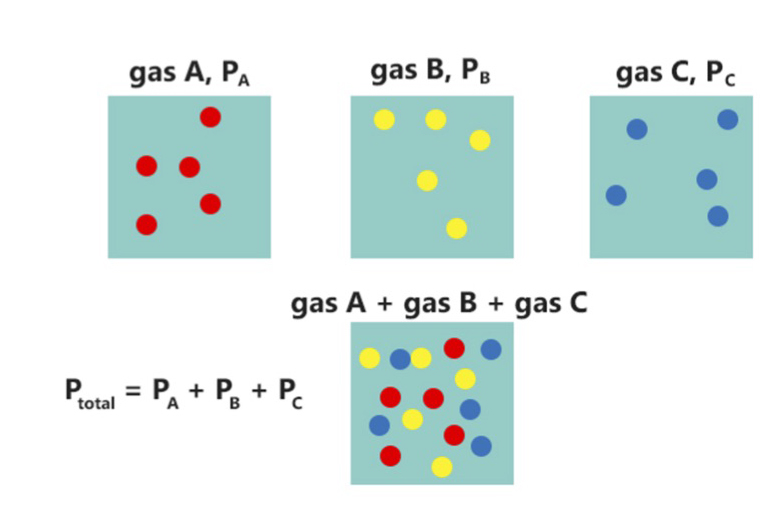
Partial Pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture ( Dalton's Law). The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dry Distillation
Dry distillation is the heating of solid materials to produce gaseous products (which may condense into liquids or solids). The method may involve pyrolysis or thermolysis, or it may not (for instance, a simple mixture of ice and glass could be separated without breaking any chemical bonds, but organic matter contains a greater diversity of molecules, some of which are likely to break). If there are no chemical changes, just phase changes, it resembles classical distillation, although it will generally need higher temperatures. Dry distillation in which chemical changes occur is a type of destructive distillation or cracking. Uses The method has been used to obtain liquid fuels from coal and wood. It can also be used to break down mineral salts such as sulfates () through thermolysis, in this case producing sulfur dioxide (SO2) or sulfur trioxide (SO3) gas which can be dissolved in water to obtain sulfuric acid. By this method sulfuric acid was first identified and artificial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Explosive Boiling Or Phase Explosion
In thermodynamics, explosive boiling or phase explosion is a method whereby a superheated metastable liquid undergoes an explosive liquid-vapor phase transition into a stable two-phase state because of a massive homogeneous nucleation of vapor bubbles. This concept was pioneered by M. M. Martynyuk in 1976 and then later advanced by Fucke and Seydel. Mechanism Explosive boiling can be best described by a p-T phase diagram. Figure on right shows a typical p-T phase diagram of a substance. The binodal line or the coexistence curve is a thermodynamic state where at that specific temperature and pressure, liquid and vapor can coexist. The spinodal line on right is the boundary of absolute instability of a solution to decomposition into multiple phases. A typical heating process is shown using red ink. If the heating process is relatively slow, the liquid has enough time to relax to an equilibrium state and the liquid follows the binodal curve, the Clausius–Clapeyron relation is st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Area
Area is the quantity that expresses the extent of a region on the plane or on a curved surface. The area of a plane region or ''plane area'' refers to the area of a shape or planar lamina, while '' surface area'' refers to the area of an open surface or the boundary of a three-dimensional object. Area can be understood as the amount of material with a given thickness that would be necessary to fashion a model of the shape, or the amount of paint necessary to cover the surface with a single coat. It is the two-dimensional analogue of the length of a curve (a one-dimensional concept) or the volume of a solid (a three-dimensional concept). The area of a shape can be measured by comparing the shape to squares of a fixed size. In the International System of Units (SI), the standard unit of area is the square metre (written as m2), which is the area of a square whose sides are one metre long. A shape with an area of three square metres would have the same area as three such s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Energy
The term "thermal energy" is used loosely in various contexts in physics and engineering. It can refer to several different well-defined physical concepts. These include the internal energy or enthalpy of a body of matter and radiation; heat, defined as a type of energy transfer (as is thermodynamic work); and the characteristic energy of a degree of freedom, k_T, in a system that is described in terms of its microscopic particulate constituents (where T denotes temperature and k_ denotes the Boltzmann constant). Relation to heat and internal energy In thermodynamics, heat is energy transferred to or from a thermodynamic system by mechanisms other than thermodynamic work or transfer of matter, such as conduction, radiation, and friction. Heat refers to a quantity transferred between systems, not to a property of any one system, or "contained" within it. On the other hand, internal energy and enthalpy are properties of a single system. Heat and work depend on the way in which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Of Vaporization
The enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that substance into a gas. The enthalpy of vaporization is a function of the pressure at which that transformation takes place. The enthalpy of vaporization is often quoted for the normal boiling temperature of the substance. Although tabulated values are usually corrected to 298 K, that correction is often smaller than the uncertainty in the measured value. The heat of vaporization is temperature-dependent, though a constant heat of vaporization can be assumed for small temperature ranges and for reduced temperature T_r \ll 1. The heat of vaporization diminishes with increasing temperature and it vanishes completely at a certain point called the critical temperature (T_r = 1). Above the critical temperature, the liquid and vapor phases are indistinguishab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of a liquid varies depending upon the surrounding environmental pressure. A liquid in a partial vacuum has a lower boiling point than when that liquid is at atmospheric pressure. A liquid at low pressure has a lower boiling point than when that liquid is at atmospheric pressure. Because of this, water boils at under standard pressure at sea level, but at at altitude. For a given pressure, different liquids will boil at different temperatures. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. At that temperature, the vapor pressure of the liquid becomes sufficient to overcome atmosphe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |