|
Golgi Matrix
The Golgi matrix is a collection of proteins involved in the structure and function of the Golgi apparatus. The matrix was first isolated in 1994 as an amorphous collection of 12 proteins that remained associated together in the presence of Triton X-100, detergent (which removed Golgi membranes) and 150 Milli-, mMolar concentration, M NaCl (which removed weakly associated proteins). Treatment with a Proteinase K, protease enzyme removed the matrix, which confirmed the importance of proteins for the matrix structure. Modern Electron microscope#Sample preparation, freeze etch electron microscopy (EM) clearly shows a mesh connecting Golgi cisternae and associated Vesicle (biology and chemistry), vesicles. Further support for the existence of a matrix comes from EM images showing that ribosomes are excluded from regions between and near Golgi cisternae.Fig. 14 in Structure and function The first individual protein component of the matrix was identified in 1995 as Golgin A2 (then cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
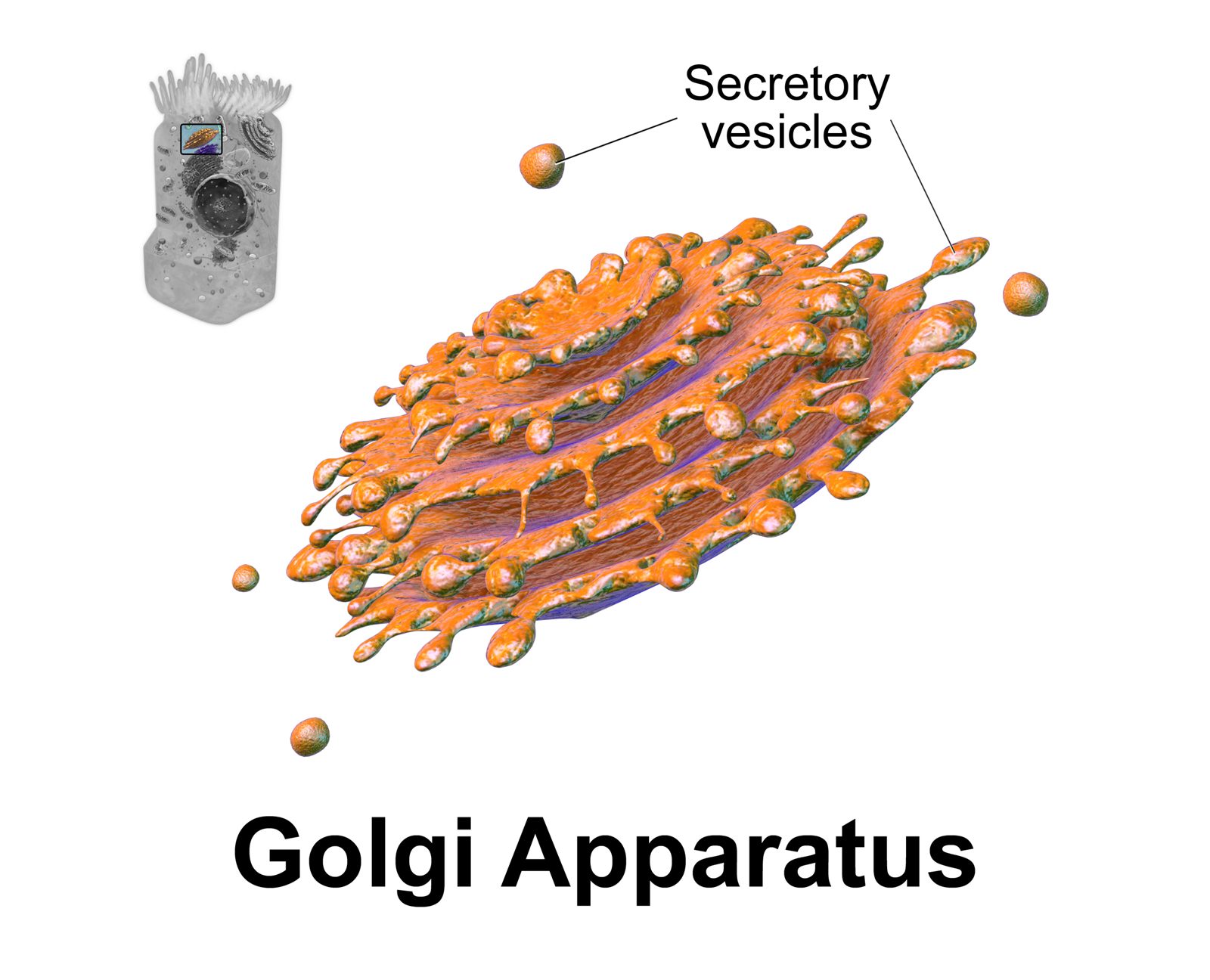
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ARF1
ADP-ribosylation factor 1 is a protein that in humans is encoded by the ''ARF1'' gene. Function ADP-ribosylation factor 1 (ARF1) is a member of the human ARF gene family. The family members encode small guanine nucleotide-binding proteins that stimulate the ADP-ribosyltransferase activity of cholera toxin and play a role in vesicular trafficking as activators of phospholipase D. The gene products, including 6 ARF proteins and 11 ARF-like proteins, constitute a family of the RAS superfamily. The ARF proteins are categorized as class I (ARF1, ARF2 and ARF3), class II (ARF4 and ARF5) and class III (ARF6), and members of each class share a common gene organization. The ARF1 protein is localized to the Golgi apparatus and has a central role in intra-Golgi transport. Multiple alternatively spliced transcript variants encoding the same protein have been found for this gene. The major mechanism of action of Brefeldin A is through inhibition of ARF1. Interactions ARF1 has been sho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GCC2
GRIP and coiled-coil domain-containing protein 2 is a protein that in humans is encoded by the ''GCC2'' gene. The protein encoded by this gene is a peripheral membrane protein localized to the trans-Golgi network. It is sensitive to brefeldin A. This encoded protein contains a GRIP domain which is thought to be used in targeting. Two alternatively spliced transcript variants encoding different isoforms have been described for this gene. References Further reading * * * * * * * * * * * * * * {{gene-2-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GCC1
GRIP and coiled-coil domain-containing protein 1 is a protein that in humans is encoded by the ''GCC1'' gene. Function The protein encoded by this gene is a peripheral membrane protein. It is sensitive to brefeldin A. This encoded protein contains a GRIP domain which is thought to be used in targeting. It may play a role in the organization of trans-Golgi network subcompartment involved with membrane transport. Interactions GCC1 has been shown to interact with TRIM29 Tripartite motif-containing protein 29 is a protein that in humans is encoded by the ''TRIM29'' gene. Function The protein encoded by this gene belongs to the TRIM protein family. It has multiple zinc finger motifs and a leucine zipper motif. .... References Further reading * * * * * {{gene-7-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COH1
Intermembrane lipid transfer protein VPS13B, also known as vacuolar protein sorting-associated 13B, and Cohen syndrome protein 1 is a protein that in humans is encoded by the ''VPS13B'' gene. It is a giant protein associated with the Golgi apparatus that is believed to be involved in post-Golgi apparatus sorting and trafficking. Mutations in the human VPS13B gene cause Cohen syndrome. ''VPS13B'' gene is also referred to as CHS1, COH1, KIAA0532, and DKFZp313I0811. The cytogenetic location of the human VPS13B gene is 8q22, which is the long arm of chromosome eight at position 22.2. Various splice variants encoding isoforms have been identified. The canonical form of the expressed protein encoded by the human VPS13B gene has 3997 amino acids. Gene The VPS13B gene is located on chromosome 8q22. Deletions in this chromosome are associated with Cohen syndrome, which is why this gene is alternatively called COH1. The gene is made up of 66 exons, four of which are alternative. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AKAP9
A-kinase anchor protein 9 is a protein that in humans is encoded by the ''AKAP9'' gene. AKAP9 is also known as Centrosome- and Golgi-localized protein kinase N-associated protein (CG-NAP) or AKAP350 or AKAP450 Function The A-kinase anchor proteins (AKAPs) are a group of structurally diverse proteins which have the common function of binding to the regulatory subunit of protein kinase A (PKA) and confining the holoenzyme to discrete locations within the cell. This gene encodes a member of the AKAP family. Alternate splicing of this gene results in many isoforms that localize to the centrosome and the Golgi apparatus, and interact with numerous signaling proteins from multiple signal transduction pathways. These signaling proteins include type II protein kinase A, serine/threonine kinase protein kinase N, protein phosphatase 1, protein phosphatase 2a, protein kinase C-epsilon and phosphodiesterase 4D3. Model organisms Model organisms have been used in the study of AKAP9 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CUTL1
Cux1 (CUTL1, CDP, CDP/Cux) is a homeodomain protein that in humans is encoded by the ''CUX1'' gene. Function The protein encoded by this gene is a member of the homeodomain family of DNA binding proteins. It regulates gene expression, morphogenesis, and differentiation and it also plays a role in cell cycle progression, particularly at S-phase. Several alternatively spliced transcript variants of this gene have been described, but the full-length nature of some of these variants has not been determined, and the p200 isoform of Cux1 is processed proteolytically to smaller active isoforms, such as p110. Cux1 DNA binding is stimulated by activation of the PAR2/F2RL1 cell-surface G-protein-coupled receptor in fibroblasts and breast-cancer epithelial cells to regulate Matrix metalloproteinase 10, Interleukin1-alpha, and Cyclo-oxygenase 2 (COX2) genes. Role in tumor growth Genetic data from over 7,600 cancer patients shows that over 1% has the deactivated CUX1 which links to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BICD1
Bicaudal D cargo adaptor 1 is a protein that in humans is encoded by the ''BICD1'' gene. Function This gene is one of two human homologs of Drosophila bicaudal-D. It has been implicated in COPI-independent membrane transport from the Golgi apparatus to the endoplasmic reticulum. Two alternative splice variants have been described. Other alternative splice variants that encode different protein isoforms have been described but their full-length nature has not been determined. Interactions BICD1 has been shown to interact with RAB6A Ras-related protein Rab-6A is a protein that in humans is encoded by the ''RAB6A'' gene located in the eleventh chromosome. Its main function is the regulation of protein transport from the Golgi complex to the endoplasmic reticulum and the exocyto .... References External links * Further reading * * * * * * * * * * * {{gene-12-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myristic Acid
Myristic acid (IUPAC name: tetradecanoic acid) is a common saturated fatty acid with the molecular formula CH3(CH2)12COOH. Its salts and esters are commonly referred to as myristates or tetradecanoates. It is named after the binomial name for nutmeg ('' Myristica fragrans''), from which it was first isolated in 1841 by Lyon Playfair. Occurrence Nutmeg butter has 75% trimyristin, the triglyceride of myristic acid. Besides nutmeg, myristic acid is found in palm kernel oil, coconut oil, butterfat, 8–14% of bovine milk, and 8.6% of breast milk as well as being a minor component of many other animal fats. It is found in spermaceti, the crystallized fraction of oil from the sperm whale. It is also found in the rhizomes of the Iris, including Orris root. Chemical behaviour Myristic acid acts as a lipid anchor in biomembranes. Reduction of myristic acid yields myristyl aldehyde Myristyl aldehyde, also known as tetradecanal, is a reduced form of myristic acid. It is na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
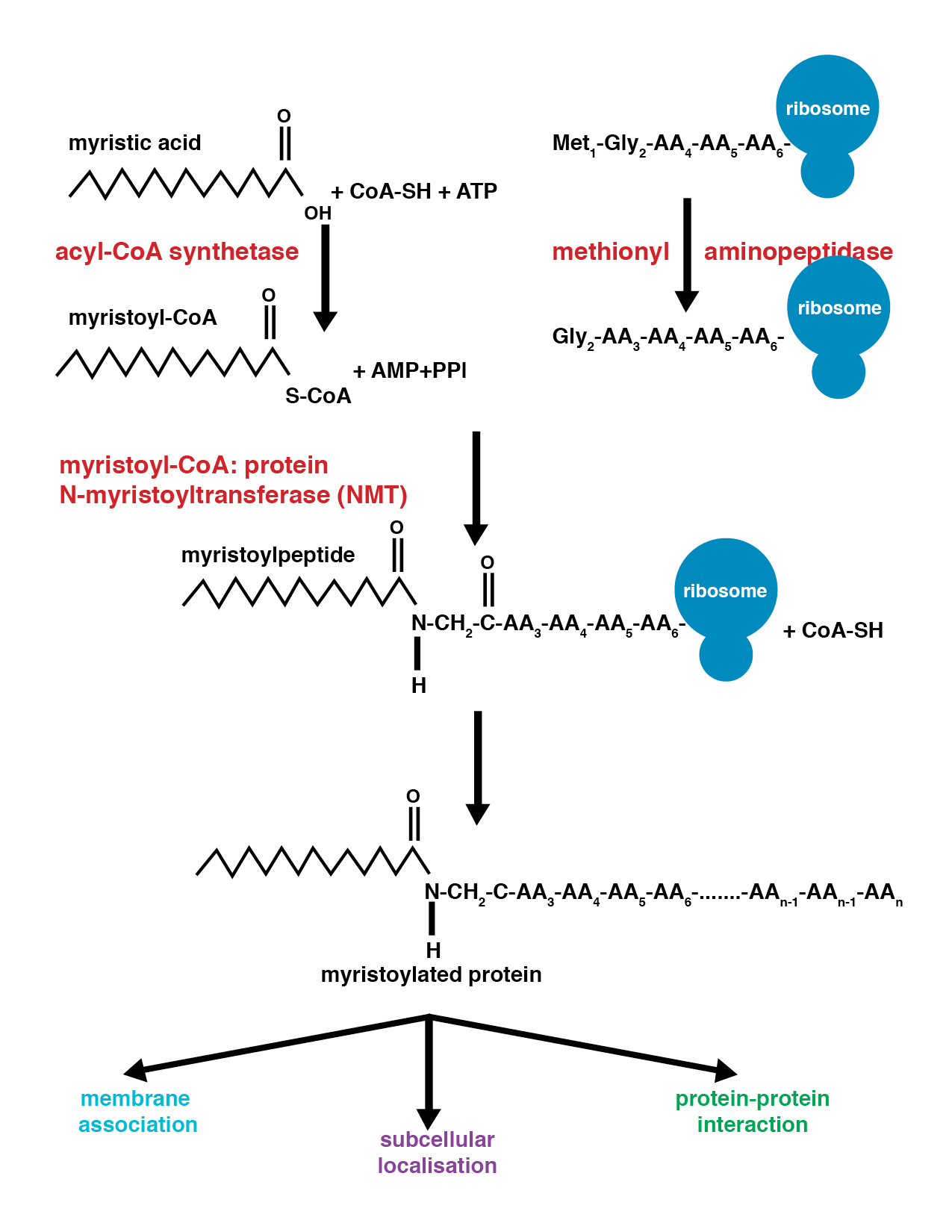
Myristoylation
Myristoylation is a lipidation modification where a myristoyl group, derived from myristic acid, is covalently attached by an amide bond to the alpha-amino group of an N-terminal glycine residue. Myristic acid is a 14-carbon saturated fatty acid (14:0) with the systematic name of ''n''-Tetradecanoic acid. This modification can be added either co-translationally or post-translationally. N-myristoyltransferase (NMT) catalyzes the myristic acid addition reaction in the cytoplasm of cells. This lipidation event is the most found type of fatty acylation and is common among many organisms including animals, plants, fungi, protozoans and viruses. Myristoylation allows for weak protein–protein and protein–lipid interactions and plays an essential role in membrane targeting, protein–protein interactions and functions widely in a variety of signal transduction pathways. Discovery In 1982, Koiti Titani's lab identified an "N-terminal blocking group" on the catalytic subunit of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GRASP55
Golgi reassembly-stacking protein of 55 k Da (GRASP55) also known as golgi reassembly-stacking protein 2 (GORASP2) is a protein that in humans is encoded by the ''GORASP2'' gene. It was identified by its homology with GRASP65 and the protein's amino acid sequence was determined by analysis of a molecular clone of its complementary DNA. The first (N-terminus) 212 amino acid residues of GRASP55 are highly homologous to those of GRASP65, but the remainder of the 454 amino acid residues are highly diverged from GRASP65. The conserved region is known as the GRASP domain, and it is conserved among GRASPs of a wide variety of eukaryotes, but not plants. The C-terminus portion of the molecule is called the ''SPR domain'' (serine, proline-rich). GRASP55 is more closely related to homologues in other species, suggesting that GRASP55 is ancestral to GRASP65. GRASP55 is found associated with the medial and trans cisternae of the Golgi apparatus. Function GRASP55 is involved in establi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GRASP65
Golgi reassembly-stacking protein of 65 kDa (GRASP65) also known as Golgi reassembly-stacking protein 1 (GORASP1) is a protein that in humans is encoded by the ''GORASP1'' gene. Function The Golgi complex plays a key role in the sorting and modification of proteins exported from the endoplasmic reticulum. The GRASP65 protein is a peripheral membrane protein anchored to the lipid bilayer through myristoylation of a glycine residue near the protein's amino terminus. It is involved in establishing the stacked structure of the Golgi apparatus and linking the stacks into larger ribbons in vertebrate cells. It is a caspase-3 substrate, and cleavage of this encoded protein contributes to Golgi fragmentation in apoptosis. GRASP65 can form a complex with the Golgi matrix protein GM130, and this complex binds to the vesicle docking protein p115. Several alternatively spliced transcript variants of this gene have been identified, but their full-length natures have not been determined. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |