|
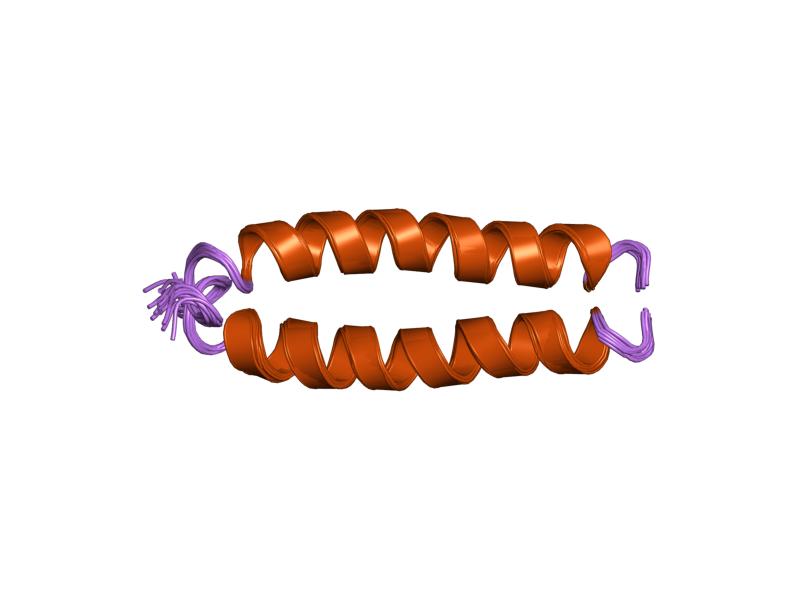
Globin X
The globins are a superfamily of heme-containing globular proteins, involved in binding and/or transporting oxygen. These proteins all incorporate the globin fold, a series of eight alpha helical segments. Two prominent members include myoglobin and hemoglobin. Both of these proteins reversibly bind oxygen via a heme prosthetic group. They are widely distributed in many organisms. Structure Globin superfamily members share a common three-dimensional fold. This 'globin fold' typically consists of eight alpha helices, although some proteins have additional helix extensions at their termini. Since the globin fold contains only helices, it is classified as an all-alpha protein fold. The globin fold is found in its namesake globin families as well as in phycocyanins. The globin fold was thus the first protein fold discovered (myoglobin was the first protein whose structure was solved). Helix packaging The eight helices of the globin fold core share significant nonlocal st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Superfamily
A protein superfamily is the largest grouping (clade) of proteins for which common ancestry can be inferred (see homology (biology), homology). Usually this common ancestry is inferred from structural alignment and mechanistic similarity, even if no sequence similarity is evident. Sequence homology can then be deduced even if not apparent (due to low sequence similarity). Superfamilies typically contain several protein families which show sequence similarity within each family. The term ''protein clan'' is commonly used for protease and glycosyl hydrolases superfamilies based on the MEROPS and CAZy classification systems. Identification Superfamilies of proteins are identified using a number of methods. Closely related members can be identified by different methods to those needed to group the most evolutionarily divergent members. Sequence similarity Historically, the similarity of different amino acid sequences has been the most common method of inferring Sequence homology, h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Globin X
The globins are a superfamily of heme-containing globular proteins, involved in binding and/or transporting oxygen. These proteins all incorporate the globin fold, a series of eight alpha helical segments. Two prominent members include myoglobin and hemoglobin. Both of these proteins reversibly bind oxygen via a heme prosthetic group. They are widely distributed in many organisms. Structure Globin superfamily members share a common three-dimensional fold. This 'globin fold' typically consists of eight alpha helices, although some proteins have additional helix extensions at their termini. Since the globin fold contains only helices, it is classified as an all-alpha protein fold. The globin fold is found in its namesake globin families as well as in phycocyanins. The globin fold was thus the first protein fold discovered (myoglobin was the first protein whose structure was solved). Helix packaging The eight helices of the globin fold core share significant nonlocal st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoglobin
Cytoglobin is the protein product of CYGB, a human and mammalian gene. Cytoglobin is a globin molecule ubiquitously expressed in all tissues and most notably utilized in marine mammals. It was discovered in 2001 in hepatic stellate cells during liver fibrosis. Thus, it was originally called "stellate cell activated protein" or STAP. It received its current name in 2002. It is thought to help in the distribution and storage of oxygen as well as protect against hypoxia by scavenging reactive oxygen species . The predicted function of cytoglobin is the facilitation of oxygen among tissues that don't express myoglobin. Function Cytoglobin is a ubiquitously expressed hexacoordinate hemoglobin that may facilitate diffusion of oxygen through tissues, scavenge nitric oxide or reactive oxygen species, or serve a protective function during oxidative stress. Structure Cytoglobin has 30-40% sequence homology with myoglobin, and has a similar oxygen binding affinity. One of the major ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even though the domain Archaea Cladistics, cladistically includes eukaryotes, the term "archaea" (: archaeon , from the Greek "ἀρχαῖον", which means ancient) in English still generally refers specifically to prokaryotic members of Archaea. Archaea were initially Taxonomy (biology), classified as bacteria, receiving the name archaebacteria (, in the Archaebacteria Kingdom (biology), kingdom), but this term has fallen out of use. Archaeal cells have unique properties separating them from Bacteria and Eukaryote, Eukaryota. Archaea are further divided into multiple recognized phylum, phyla. Classification is difficult because most have not been Isolation (microbiology), isolated in a laboratory and have been detected only by their Gene, gene s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytoglobin
Phytoglobins are globular plant (algae and land plant) proteins classified into the globin superfamily, which contain a heme, ''i''.''e''. protoporphyrin IX-Fe, prosthetic group. The earliest known phytoglobins are leghemoglobins, discovered in 1939 by Kubo after spectroscopic and chemical analysis of the red pigment of soybean root nodules. A few decades after Kubo's report the crystallization of a lupin phytoglobin (known as leghemoglobin) by Vainshtein and collaborators revealed that the tertiary structure of this protein and that of the sperm whale myoglobin was remarkably similar, thus indicating that the phytoglobin discovered by Kubo did indeed correspond to a globin. One important function of phytoglobin is its nitric oxide dioxygenase activity. Distribution and classification Phytoglobins (abbreviated as Phytogbs) are ubiquitously distributed in both green algae and land plants. They can be classified as follows: – Phylogeny is from 2007 and quite rough. This art ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fusion Protein
Fusion proteins or chimeric (kī-ˈmir-ik) proteins (literally, made of parts from different sources) are proteins created through the joining of two or more genes that originally coded for separate proteins. Translation of this '' fusion gene'' results in a single or multiple polypeptides with functional properties derived from each of the original proteins. ''Recombinant fusion proteins'' are created artificially by recombinant DNA technology for use in biological research or therapeutics. '' Chimeric'' or ''chimera'' usually designate hybrid proteins made of polypeptides having different functions or physico-chemical patterns. ''Chimeric mutant proteins'' occur naturally when a complex mutation, such as a chromosomal translocation, tandem duplication, or retrotransposition creates a novel coding sequence containing parts of the coding sequences from two different genes. Naturally occurring fusion proteins are commonly found in cancer cells, where they may function as oncop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |