|
Geldanamycin
Geldanamycin is a 1,4-benzoquinone ansamycin Antitumor agent, antitumor antibiotic that inhibits the function of Hsp90 (Heat Shock Protein 90) by binding to the unusual ADP/ATP-binding pocket of the protein. HSP90 client proteins play important roles in the regulation of the cell cycle, cell growth, cell survival, apoptosis, angiogenesis and oncogenesis. Geldanamycin induces the degradation of proteins that are mutated or overexpressed in tumor cells such as v-Src, Bcr-Abl, p53, and ERBB2. This effect is mediated via HSP90. Despite its potent antitumor potential, geldanamycin presents several major drawbacks as a drug candidate such as hepatotoxicity, further, Jilani ''et al.''. reported that geldanamycin induces the apoptosis of erythrocytes under physiological concentrations. These side effects have led to the development of geldanamycin analogues, in particular analogues containing a derivatisation at the 17 position: * 17-AAG * 17-DMAG Biosynthesis Geldanamycin was original ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hsp90
Hsp90 (heat shock protein 90) is a chaperone (protein), chaperone protein that assists other proteins to protein folding, fold properly, stabilizes proteins against heat stress, and aids in protein degradation. It also stabilizes a number of proteins required for tumor growth, which is why Hsp90 inhibitors are investigated as anti-cancer drugs. Heat shock proteins, as a class, are among the most highly expressed cell (biology), cellular proteins across all species. As their name implies, heat shock proteins protect cells when stressed by elevated temperatures. They account for 1–2% of total protein in unstressed cells. However, when cells are heated, the fraction of heat shock proteins increases to 4–6% of cellular proteins. Heat shock protein 90 (Hsp90) is one of the most common of the heat-related proteins. The "90" comes from the fact that it has a mass of roughly 90 Atomic mass unit, kilodaltons. A 90 kDa protein is considered fairly large for a non-fibrou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
17-DMAG
17-Dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) is a chemical compound which is a semi-synthetic derivative of the antibiotic geldanamycin Geldanamycin is a 1,4-benzoquinone ansamycin Antitumor agent, antitumor antibiotic that inhibits the function of Hsp90 (Heat Shock Protein 90) by binding to the unusual ADP/ATP-binding pocket of the protein. HSP90 client proteins play important rol .... It is being studied for the possibility of treating cancer. References Antibiotics Macrocycles 1,4-Benzoquinones Carbamates Lactams Ethers Secondary alcohols Conjugated dienes {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
17-AAG
Tanespimycin (17-''N''-allylamino-17-demethoxygeldanamycin, 17-AAG) is a derivative of the antibiotic geldanamycin that is being studied in the treatment of cancer, specifically in younger patients with certain types of leukemia or solid tumors, especially kidney tumors. It works by inhibiting Hsp90, which is expressed in those tumors. It belongs to the family of drugs called antitumor antibiotics. Clinical trials Bristol-Myers Squibb conducted Phase 1 and Phase 2 clinical trials. However, in 2010 the company halted development Development or developing may refer to: Arts *Development (music), the process by which thematic material is reshaped * Photographic development *Filmmaking, development phase, including finance and budgeting * Development hell, when a proje ... of tanespimycin, during late-stage clinical trials as a potential treatment for multiple myeloma. While no definitive explanation was given, it has been suggested that Bristol-Myers Squibb halted devel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ansamycin
Ansamycins is a family of bacterial secondary metabolites that show antimicrobial activity against many Gram-positive and some Gram-negative bacteria, and includes various compounds, including streptovaricins and rifamycins. In addition, these compounds demonstrate antiviral activity towards bacteriophages and poxviruses. They are somewhat similar in structure to macrolide antibiotics, but because they have a lactam instead of a lactone, they do not belong in the class of macrolides. __TOC__ Structure They are named ansamycins (from the Latin ansa, ''handle'') because of their unique structure, which consists of an aromatic moiety bridged by an aliphatic chain. The main difference between various derivatives of ansamycins is the aromatic moiety, which can be a naphthalene ring or a naphthoquinone ring as in rifamycin and the naphthomycins. Another variation consists of benzene or a benzoquinone ring system as in geldanamycin or ansamitocin. Ansamycins were first discovere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptomyces Hygroscopicus
''Streptomyces hygroscopicus'' is a bacterial species in the genus '' Streptomyces''. It was first described by Hans Laurits Jensen in 1931. Biochemistry Cultures of different strains of ''S. hygroscopicus'' can be used to produce several chemical compounds or enzymes. Small molecules Immunosuppressants Sirolimus (also known as rapamycin) is an antifungal and immunosuppressant that has been isolated from ''S. hygroscopicus'' from soil samples from Easter Island. Ascomycin is another immunosuppressant produced by some strains of ''S. hygroscopicus;'' it has a similar structure to sirolimus and can be used to treat autoimmune diseases and skin diseases and can help prevent rejection after an organ transplant. Antibiotics The antibiotics geldanamycin, hygromycin B, nigericin, validamycin, and cyclothiazomycin are found in ''S. hygroscopicus''. Experimental cancer drugs Indolocarbazoles can be found in ''S. hygroscopicus'' . Anthelmintics and insecticides Mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ERBB2
Receptor tyrosine-protein kinase erbB-2 is a protein that normally resides in the membranes of cells and is encoded by the ''ERBB2'' gene. ERBB is abbreviated from erythroblastic oncogene B, a gene originally isolated from the avian genome. The human protein is also frequently referred to as HER2 (human epidermal growth factor receptor 2) or CD340 ( cluster of differentiation 340). HER2 is a member of the human epidermal growth factor receptor (HER/EGFR/ERBB) family. But contrary to other members of the ERBB family, HER2 does not directly bind ligand. HER2 activation results from heterodimerization with another ERBB member or by homodimerization when HER2 concentration are high, for instance in cancer. Amplification or over-expression of this oncogene has been shown to play an important role in the development and progression of certain aggressive types of breast cancer. In recent years the protein has become an important biomarker and target of therapy for approximately 30% o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethers
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group (e.g., alkyl or aryl). They have the general formula , where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol Ethers
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactams
A lactam is a cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words '' lactone'' + ''amide''. Nomenclature Greek prefixes in alphabetical order indicate ring size. This ring-size nomenclature stems from the fact that hydrolysis of an α-lactam gives an α-amino acid and that of a β-Lactam gives a β-amino acid, and so on. Synthesis General synthetic methods are used for the organic synthesis of lactams. Beckmann rearrangement Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement. Schmidt reaction Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclohexanone with hydrazoic acid, forms ε - Caprolactum, which upon treatment with excess acid forms Cardiazole, a heart stimulant. Cyclization of amino acids Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
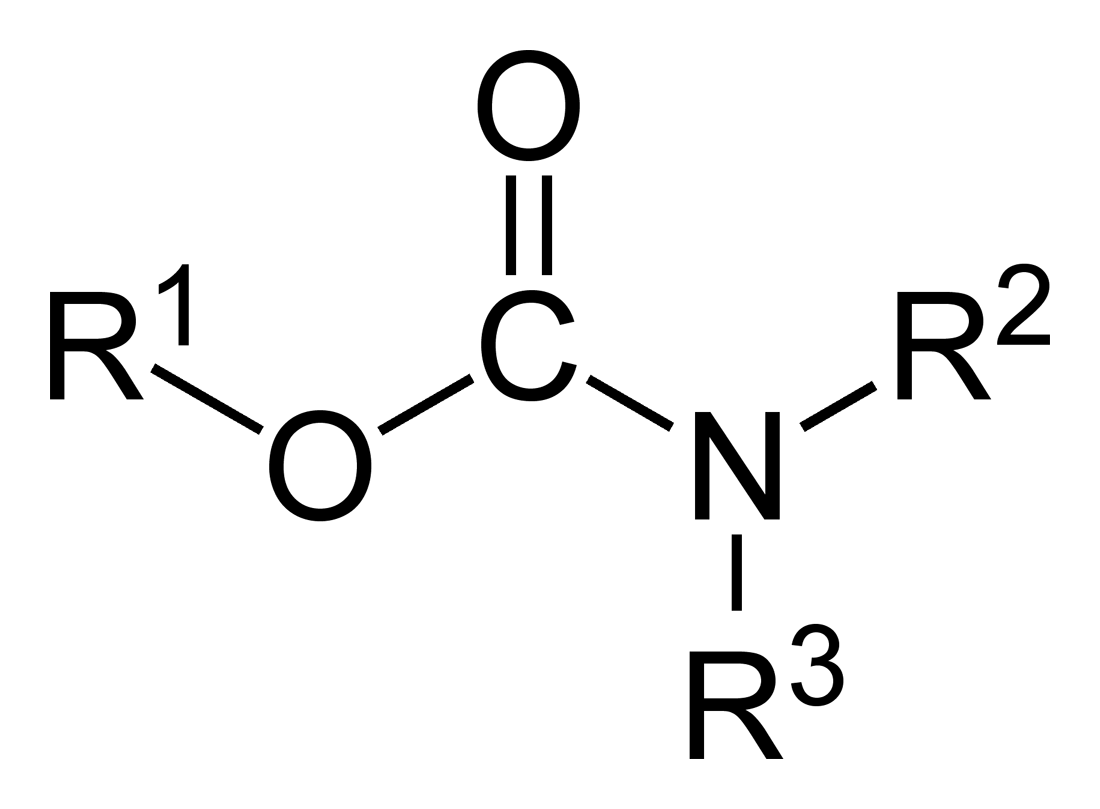
Carbamates
In organic chemistry, a carbamate is a category of organic compounds with the general formula and structure , which are formally derived from carbamic acid (). The term includes organic compounds (e.g., the ester ethyl carbamate), formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion (e.g. ammonium carbamate). Polymers whose repeat units are joined by carbamate like groups are an important family of plastics, the polyurethanes. See for clarification. Properties While carbamic acids are unstable, many carbamate esters and salts are stable and well known. Equilibrium with carbonate and bicarbonate In water solutions, the carbamate anion slowly equilibrates with the ammonium cation and the carbonate or bicarbonate anions: : : Calcium carbamate is soluble in water, whereas calcium carbonate is not. Adding a calcium salt to an ammonium carbamate/carbonate solution will precipitate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Data Bank
The Protein Data Bank (PDB) is a database for the three-dimensional structural data of large biological molecules such as proteins and nucleic acids, which is overseen by the Worldwide Protein Data Bank (wwPDB). This structural data is obtained and deposited by biologists and biochemists worldwide through the use of experimental methodologies such as X-ray crystallography, Nuclear magnetic resonance spectroscopy of proteins, NMR spectroscopy, and, increasingly, cryo-electron microscopy. All submitted data are reviewed by expert Biocuration, biocurators and, once approved, are made freely available on the Internet under the CC0 Public Domain Dedication. Global access to the data is provided by the websites of the wwPDB member organizations (PDBe, PDBj, RCSB PDB, and BMRB). The PDB is a key in areas of structural biology, such as structural genomics. Most major scientific journals and some funding agencies now require scientists to submit their structure data to the PDB. Many other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |