|
Gabriel Synthesis
The Gabriel synthesis is a chemical reaction that transforms primary alkyl halides into primary amines. Traditionally, the reaction uses potassium phthalimide. The reaction is named after the German chemist Siegmund Gabriel. The Gabriel reaction has been generalized to include the alkylation of sulfonamides and imides, followed by deprotection, to obtain amines (see Alternative Gabriel reagents). The alkylation of ammonia is often an unselective and inefficient route to amines. In the Gabriel method, phthalimide anion is employed as a surrogate of H2N−. Traditional Gabriel synthesis In this method, the sodium or potassium salt of phthalimide is ''N''-alkylated with a primary alkyl halide to give the corresponding ''N''-alkylphthalimide. Upon workup by acidic hydrolysis the primary amine is liberated as the amine salt. Alternatively the workup may be via the Ing–Manske procedure, involving reaction with hydrazine. This method produces a precipitate of phthalhydrazide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siegmund Gabriel
Siegmund Gabriel (7 November 1851 – 22 March 1924) was a German chemist. Scientific career Siegmund Gabriel began studying chemistry at the University of Berlin in 1871. He continued his studies at the University of Heidelberg in 1872 with Professor Robert Bunsen, Robert Wilhelm Bunsen. In 1874, he received his doctorate and then returned to Berlin. He began teaching as an assistant, initially in the inorganic chemistry department, before becoming an associate professor in 1886. Gabriel later turned to organic chemistry in his own research. One of Gabriel’s most significant contributions to organic chemistry was made in 1887, when he discovered the Gabriel synthesis, Gabriel Synthesis with his partner James Dornbush. The Gabriel Synthesis is a reaction which synthesizes pure Amine, primary amines, involving the reaction of potassium phthalimide with an Haloalkane, alkyl halide, followed by hydrolysis. The Gabriel Synthesis was adapted by Gabriel in 1889 to a procedure f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gabriel Synthesis Scheme
In the Abrahamic religions (Judaism, Christianity, Islam), Gabriel ( ) is an archangel with the power to announce God's will to mankind, as the messenger of God. He is mentioned in the Hebrew Bible, the New Testament and the Quran. Many Christian traditions – including Eastern Orthodoxy, Catholicism, Lutheranism, and Anglicanism – revere Gabriel as a saint. In the Hebrew Bible, Gabriel appears to the prophet Daniel to explain his visions ( Daniel 8:15–26, 9:21–27). The archangel also appears in the Book of Enoch and other ancient Jewish writings not preserved in Hebrew. Alongside the archangel Michael, Gabriel is described as the guardian angel of the people of Israel, defending it against the angels of the other peoples. In the New Testament, the Gospel of Luke relates the Annunciation, in which the angel Gabriel appears to Zechariah foretelling the birth of John the Baptist with the angel Gabriel foretelling the Virgin Mary the birth of Jesus Christ, res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Delépine Reaction
The Delépine reaction is the organic synthesis of primary amines (4) by reaction of benzyl or alkyl halides (1) with hexamethylenetetramine (2) followed by acid hydrolysis of the quaternary ammonium salt (3). It is named after the French chemist Stéphane Marcel Delépine (1871–1965). Advantages of this reaction are selective access to the primary amine without side reactions from easily accessible reactants with short reaction times and relatively mild reaction conditions. Downsides include that the reaction is often performed using chloroform as solvent, which is toxic, and poor atom economy, including the formation of several equivalents of formaldehyde (a known carcinogen) during quaternary ammonium salt formation. An example is the synthesis of 2-bromoallylamine from 2,3-dibromopropene. Reaction mechanism The benzyl halide or alkyl halide 1 reacts with hexamethylenetetramine to a quaternary ammonium salt 3, each time just alkylating one nitrogen atom. By refluxing in conc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robinson–Gabriel Synthesis
The Robinson–Gabriel synthesis is an organic reaction in which a 2-acylamino-ketone reacts intramolecularly followed by a dehydration to give an oxazole. A cyclodehydrating agent is needed to catalyze the reaction It is named after Robert Robinson (organic chemist), Sir Robert Robinson and Siegmund Gabriel who described the reaction in 1909 and 1910, respectively. The 2-acylamino-ketone starting material can be synthesized using the Dakin–West reaction. Modifications Recently, a Solid-phase synthesis, solid-phase version of the Robinson–Gabriel synthesis has been described. The reaction requires trifluoroacetic anhydride to be used as the cyclodehydrating agent in ether, ethereal solvent and the 2-acylamidoketone be linked by the nitrogen atom to a benzhydrylic-type linker. A one-pot diversity-oriented synthesis has been developed via a Friedel-Crafts/Robinson–Gabriel synthesis using a general oxazolone template. The combination of aluminum chloride as the Friedel–Cra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl Halides
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
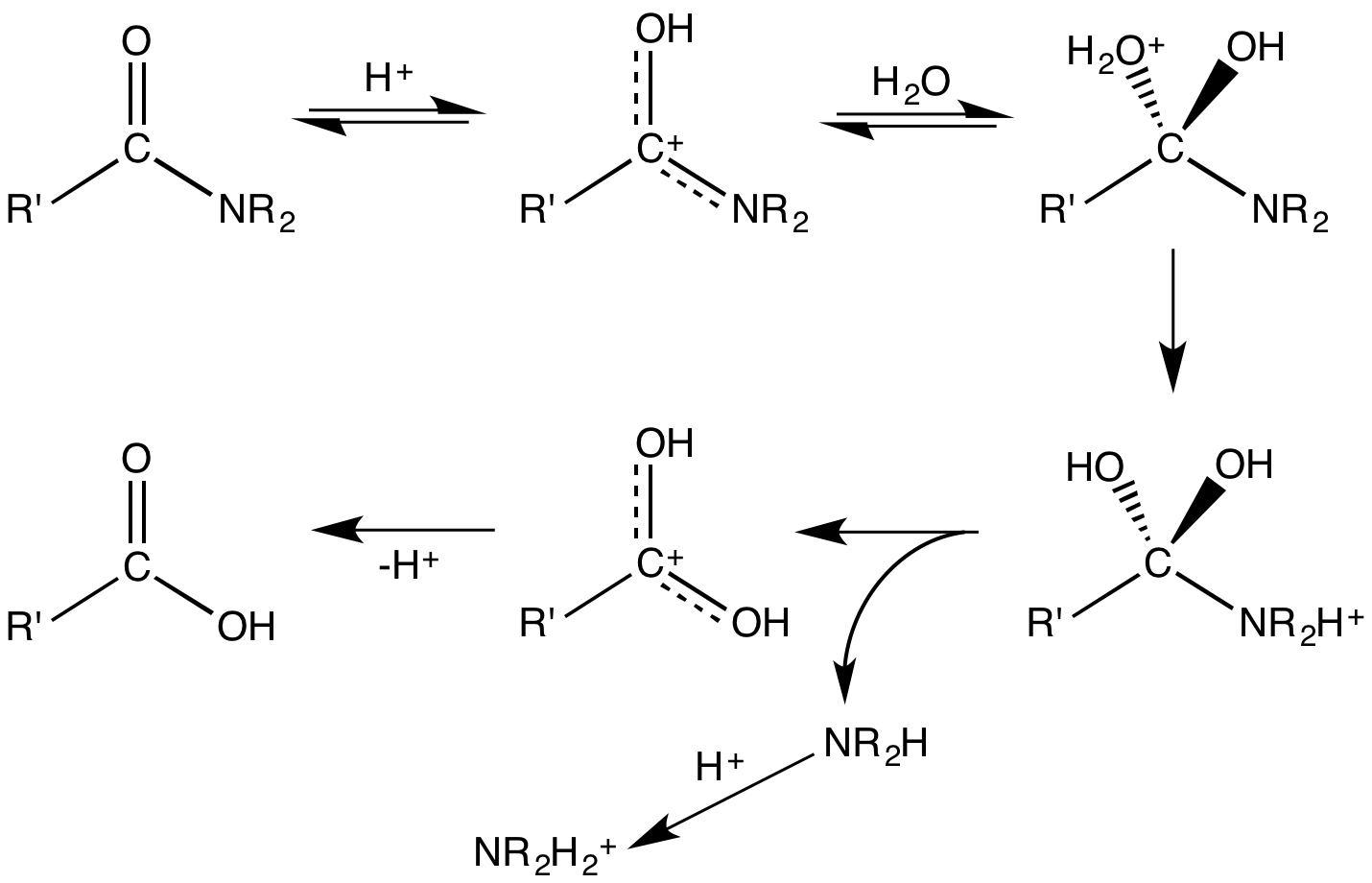
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are Lewis bases. ''Nucleophilic'' describes the affinity of a nucleophile to bond with positively charged Atomic nucleus, atomic nuclei. Nucleophilicity, sometimes referred to as nucleophile strength, refers to a substance's nucleophilic character and is often used to compare the affinity of atoms. Neutral nucleophilic reactions with solvents such as Alcohol (chemistry), alcohols and water are named solvolysis. Nucleophiles may take part in nucleophilic substitution, whereby a nucleophile becomes attracted to a full or partial positive charge, and nucleophilic addition. Nucleophilicity is closely related to basicity. The difference between the two is, that basicity is a thermodynamic property (i.e. relates to an equilibrium state), but nucleop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Di-tert-butyl-iminodicarboxylate
Di-''tert''-butyl-iminodicarboxylate is an organic compound that can be described with the formula CH3)3COC(O)sub>2NH. It is a white solid that is soluble in organic solvents. The compound is used as a reagent for the preparation of primary amines from alkyl halides. It was popularized as an alternative to the Gabriel synthesis for the same conversion. Amines can also be prepared from alcohols by dehydration using the Mitsunobu reaction.Neelamkavil, Santhosh "Di-tert-butyl-imidocarbonate" e-EROS Encyclopedia of Reagents for Organic Synthesis, 2001, John Wiley & Sons. {{doi, 10.1002/047084289X.rn00488 In the usual implementation the reagent is deprotonated Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ... to give the potassium salt, which is ''N''-alkylated. The Boc protecting gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saccharin
Saccharin, also called saccharine, benzosulfimide, or E954, or used in saccharin sodium or saccharin calcium forms, is a non-nutritive artificial sweetener. Saccharin is a sultam that is about 500 times sweeter than sucrose, but has a bitter or metallic aftertaste, especially at high concentrations. It is used to sweeten products, such as drinks, candies, baked goods, tobacco products, excipients, and for masking the bitter taste of some medicines. It appears as white crystals and is odorless. Etymology Saccharin derives its name from the word "saccharine", meaning "sugary". The word saccharine is used figuratively, often in a derogative sense, to describe something "unpleasantly over-polite" or "overly sweet". Both words are derived from the Greek word (''sakkharon'') meaning "gravel". Similarly, saccharose is an obsolete name for sucrose (table sugar). Properties Saccharin is heat-stable. It does not react chemically with other food ingredients; as such, it stores we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron Letters
''Tetrahedron Letters'' is a weekly international journal for rapid publication of full original research papers in the field of organic chemistry. According to the ''Journal Citation Reports'', the journal has a 2022 impact factor of 1.8 Indexing ''Tetrahedron Letters'' is indexed in: References See also *''Tetrahedron In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...'' *'' Tetrahedron: Asymmetry'' Chemistry journals Weekly journals Academic journals established in 1959 Elsevier academic journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing Polymeric foam, polymer foams, but applications also include its uses as a precursor (chemistry), precursor to pharmaceuticals and agrochemicals, as well as a long-term storable propellant for in-outer space, space spacecraft propulsion. Additionally, hydrazine is used in various rocket propellant, rocket fuels and to prepare the gas precursors used in airbags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. , approximately 120,000 tons of hydrazine hydrate (corresponding to a 64% solution of hydrazine in water by weight) we ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |