|
Farnesyl
Farnesol is a natural 15-carbon organic compound which is an acyclic sesquiterpene alcohol. Under standard conditions, it is a colorless liquid. It is hydrophobic, and thus insoluble in water, but miscible with oils. Farnesol is produced from 5-carbon isoprene compounds in both plants and animals. Phosphate-activated derivatives of farnesol are the building blocks of possibly all acyclic sesquiterpenoids. These compounds are doubled to form 30-carbon squalene, which is the precursor for steroids in plants, animals, and fungi. Farnesol and its derivatives are important starting compounds for natural and artificial organic synthesis. Uses Farnesol is present in many essential oils such as citronella, neroli, cyclamen, lemon grass, tuberose, rose, musk, balsam, and tolu. It is used in perfumery to emphasize the odors of sweet, floral perfumes. It enhances perfume scent by acting as a co-solvent that regulates the volatility of the odorants. It is especially used in lilac perf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Farnesyl Pyrophosphate
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids. It is also used in the synthesis of CoQ (part of the electron transport chain), as well as dehydrodolichol diphosphate (a precursor of dolichol, which transports proteins to the ER lumen for ''N''-glycosylation). Biosynthesis Farnesyl pyrophosphate synthase (a prenyl transferase) catalyzes sequential condensation reactions of dimethylallyl pyrophosphate with 2 units of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate, as is shown in the following two steps: * Dimethylallyl pyrophosphate reacts with 3-isopentenyl pyrophosphate to form geranyl pyrophosphate: * Geranyl pyrophosphate then reacts with another molecule of 3-isopentenyl pyrophosphate to form farnesyl pyrophosphate Pharmacology The above reactions are inhibited by bisphosphonates (used for osteoporosis). Farnesyl pyrophosphate is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sesquiterpene
Sesquiterpenes are a class of terpenes that consist of three isoprene units and often have the molecular formula C15H24. Like monoterpenes, sesquiterpenes may be cyclic or contain rings, including many unique combinations. Biochemical modifications such as oxidation or rearrangement produce the related sesquiterpenoids. Sesquiterpenes are found naturally in plants and insects, as semiochemicals, e.g. defensive agents or pheromones. Biosynthesis and examples The reaction of geranyl pyrophosphate with isopentenyl pyrophosphate results in the 15-carbon farnesyl pyrophosphate (FPP), which is an intermediate in the biosynthesis of sesquiterpenes such as farnesene. Cyclic sesquiterpenes are more common than cyclic monoterpenes because of the increased chain length and additional double bond in the sesquiterpene precursors. In addition to common six-membered ring systems such as the ones found in zingiberene and bisacurone, cyclization of one end of the chain to the other end can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroid
A steroid is a biologically active organic compound with four rings arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Hundreds of steroids are found in plants, animals and fungi. All steroids are manufactured in cells from the sterols lanosterol ( opisthokonts) or cycloartenol (plants). Lanosterol and cycloartenol are derived from the cyclization of the triterpene squalene. The steroid core structure is typically composed of seventeen carbon atoms, bonded in four " fused" rings: three six-member cyclohexane rings (rings A, B and C in the first illustration) and one five-member cyclopentane ring (the D ring). Steroids vary by the functional groups attached to this four-ring core and by the oxidation state of the rings. Sterols are forms of steroids with a hydroxy group at position three and a skeleton derived from cholesta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geranyl Pyrophosphate
Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoid geraniol. Its salts are colorless. It is a precursor to many natural products. Occurrence GPP is an intermediate in the isoprenoid biosynthesis pathway that produces longer prenyl chains such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate as well as many terpenes. It can be prepared in the laboratory from geraniol. Related compounds * Geraniol * Farnesyl pyrophosphate * Geranylgeranyl pyrophosphate See also * Dimethylallyltranstransferase Dimethylallyltranstransferase (DMATT), also known as farnesylpyrophosphate synthase (FPPS) or as farnesyldiphosphate synthase (FDPS), is an enzyme that in humans is encoded by the FDPS gene and catalyzes the transformation of dimethylallylpyr ... References Further reading *Kulkarni RS, Pandit SS, Chidley HG, Nagel R, Schmidt A, Gershenzon J, Pujari KH, Giri AP and Gupta VS, 2013Characterization of thre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Squalene
Squalene is an organic compound. It is a triterpenoid with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as ''Squalus'' is a genus of sharks). All plants and animals produce squalene as a biochemical intermediate to sterol biosynthesis. An estimated 12% of bodily squalene in humans is found in sebum. Squalene has a role in topical skin lubrication and protection. Squalene is a precursor for synthesis of all plant and animal sterols, including cholesterol and steroid hormones in the human body. Squalene is an important ingredient in some vaccine adjuvants: The Novartis and GlaxoSmithKline adjuvants are called MF59 and AS03, respectively. Role in steroid synthesis Squalene is the biochemical precursor to steroids. The squalene conversion begins with oxidation (via squalene monooxygenase) of one of its terminal double bonds, resulting in 2,3-oxidosqualene. It then undergoes an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linalool
Linalool () refers to two enantiomers of a naturally occurring terpene alcohol found in many flowers and spice plants. Linalool has multiple commercial applications, the majority of which are based on its pleasant scent (floral, with a touch of spiciness). A colorless oil, linalool is classified as an acyclic monoterpenoid. In plants, it is a metabolite, a volatile oil component, an antimicrobial agent, and an aroma compound. Linalool has uses in manufacturing of soaps, fragrances, food additives as flavors, household products, and insecticides. Esters of linalool are referred to as linalyl, e.g. linalyl pyrophosphate, an isomer of geranyl pyrophosphate. The word ''linalool'' is based on ''linaloe'' (a type of wood) and the suffix '. In food manufacturing, it may be called ''coriandrol''. Occurrence Both enantiomeric forms are found in nature: (''S'')-linalool is found, for example, as a major constituent of the essential oils of coriander ('' Coriandrum sativum'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
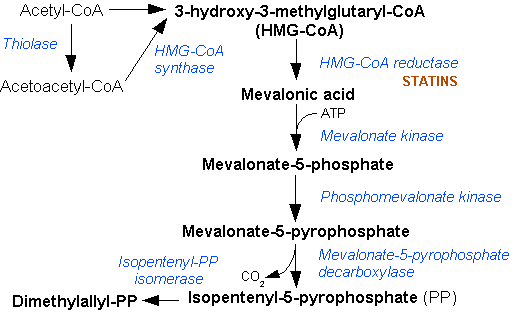
Isopentenyl Pyrophosphate
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP) is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the ''non-mevalonate'' MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids. Biosynthesis IPP is formed from acetyl-CoA via the mevalonate pathway (the "upstream" part), and then is isomerized to dimethylallyl pyrophosphate by the enzyme isopentenyl pyrophosphate isomerase. IPP can be synthesised via an alternative non-mevalonate pathway of isoprenoid precursor biosynthesis, the MEP pathway, where it is formed from (''E'')-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by the enzyme HMB-PP reductase (LytB, IspH). The MEP pathway is present in many bacteria, apicomplexan protozoa such as malaria parasites, and in the plastids of higher pla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Farnesene
The term farnesene refers to a set of six closely related chemical compounds which all are sesquiterpenes. α-Farnesene and β-farnesene are isomers, differing by the location of one double bond. α-Farnesene is 3,7,11-trimethyl-1,3,6,10-dodecatetraene and β-farnesene is 7,11-dimethyl-3-methylene-1,6,10-dodecatriene. The alpha form can exist as four stereoisomers that differ about the geometry of two of its three internal double bonds (the stereoisomers of the third internal double bond are identical). The beta isomer exists as two stereoisomers about the geometry of its central double bond. Two of the α-farnesene stereoisomers are reported to occur in nature. (''E'',''E'')-α-Farnesene is the most common isomer. It is found in the coating of apples, and other fruits, and it is responsible for the characteristic green apple odour. Its oxidation by air forms compounds that are damaging to the fruit. The oxidation products injure cell membranes which eventually causes cell d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Liv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vachellia Farnesiana
''Vachellia farnesiana'', also known as ''Acacia farnesiana'', and previously ''Mimosa farnesiana'', commonly known as sweet acacia, huisache, or needle bush, is a species of shrub or small tree in the legume family, Fabaceae. Its flowers are used in the perfume industry. Description The plant is deciduous over part of its range, but evergreen in most locales. Growing from multiple trunks, it reaches a height of . The bark is whitish gray. The base of each leaf is accompanied by a pair of thorns on the branch. The dark brown fruit is a seed pod. Taxonomy Taxonomic history It was first described by Europeans under the name ''Acacia Indica Farnesiana'' in 1625 by Tobias Aldini from plants grown in Rome in the Farnese Gardens from seed collected in Santo Domingo, in what is now the Dominican Republic, which germinated in 1611. Aldini included an illustration of the plant, which he contrasted with an illustration of the first known ''Acacia''; ''Acacia nilotica''. This first ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pheromone
A pheromone () is a secreted or excreted chemical factor that triggers a social response in members of the same species. Pheromones are chemicals capable of acting like hormones outside the body of the secreting individual, to affect the behavior of the receiving individuals. There are ''alarm pheromones'', ''food trail pheromones'', ''sex pheromones'', and many others that affect behavior or physiology. Pheromones are used by many organisms, from basic unicellular prokaryotes to complex multicellular eukaryotes. Their use among insects has been particularly well documented. In addition, some vertebrates, plants and ciliates communicate by using pheromones. The ecological functions and evolution of pheromones are a major topic of research in the field of chemical ecology. Background The portmanteau word "pheromone" was coined by Peter Karlson and Martin Lüscher in 1959, based on the Greek φερω ''pheroo'' ('I carry') and ὁρμων ''hormon'' ('stimulating'). Pheromones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |