|
Ferrous
In chemistry, iron(II) refers to the chemical element, element iron in its +2 oxidation number, oxidation state. The adjective ''ferrous'' or the prefix ''ferro-'' is often used to specify such compounds, as in ''ferrous chloride'' for iron(II) chloride (). The adjective ''ferric'' is used instead for iron(III) salts, containing the cation Fe3+. The word ''wikt:ferrous, ferrous'' is derived from the Latin word , meaning "iron". In salt (chemistry), ionic compounds (salts), such an atom may occur as a separate cation (positive ion) abbreviated as Fe2+, although more precise descriptions include other ligands such as water and halides. Iron(II) centres occur in coordination complexes, such as in the anion ferrocyanide, , where six cyanide ligands are bound the metal centre; or, in organometallic compounds, such as the ferrocene , where two cyclopentadienyl anions are bound to the FeII centre. Ferrous ions in biology All known forms of life require iron. Many proteins in living ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(II) Chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions. Production Hydrated forms of ferrous chloride are generated by treatment of wastes from steel production with hydrochloric acid. Such solutions are designated "spent acid," or "pickle liquor" especially when the hydrochloric acid is not completely consumed: :Fe + 2 HCl → FeCl2 + H2 The production of ferric chloride involves the use of ferrous chloride. Ferrous chloride is also a byproduct from the production of titanium, since some titanium ores contain iron.Egon Wildermuth, Hans Stark, Gabriele Friedrich, Franz Ludw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferric
In chemistry, iron(III) or ''ferric'' refers to the chemical element, element iron in its +3 oxidation number, oxidation state. ''Ferric chloride'' is an alternative name for iron(III) chloride (). The adjective ''ferrous'' is used instead for iron(II) salts, containing the cation Fe2+. The word ''wikt:ferric, ferric'' is derived from the Latin word , meaning "iron". Although often abbreviated as Fe3+, that naked ion does not exist except under extreme conditions. Iron(III) centres are found in many compounds and coordination complexes, where Fe(III) is bonded to several Ligand, ligands. A molecular ferric complex is the anion ferrioxalate, , with three bidentate oxalate ions surrounding the Fe core. Relative to lower oxidation states, ferric is less common in organoiron chemistry, but the ferrocenium cation is well known. Iron(III) in biology All known forms of life require iron, which usually exists in Fe(II) or Fe(III) oxidation states. Many proteins in living beings cont ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron(III)
In chemistry, iron(III) or ''ferric'' refers to the element iron in its +3 oxidation state. ''Ferric chloride'' is an alternative name for iron(III) chloride (). The adjective ''ferrous'' is used instead for iron(II) salts, containing the cation Fe2+. The word ''ferric'' is derived from the Latin word , meaning "iron". Although often abbreviated as Fe3+, that naked ion does not exist except under extreme conditions. Iron(III) centres are found in many compounds and coordination complexes, where Fe(III) is bonded to several ligands. A molecular ferric complex is the anion ferrioxalate, , with three bidentate oxalate ions surrounding the Fe core. Relative to lower oxidation states, ferric is less common in organoiron chemistry, but the ferrocenium cation is well known. Iron(III) in biology All known forms of life require iron, which usually exists in Fe(II) or Fe(III) oxidation states. Many proteins in living beings contain iron(III) centers. Examples of such metalloprot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eisen(II)-chlorid-Tetrahydrat
Eisen is a German surname meaning "iron". Notable people with the surname include: * Arnold Eisen, professor of Jewish studies * Arthur Arturovich Eisen, a Russian soloist with the Alexandrov Ensemble * Charles-Dominique-Joseph Eisen (1720–1778), French painter and engraver * Cliff Eisen, Canadian musicologist * Erez Eisen, Israeli music producer * François Eisen. French engraver * Gary Eisen, American politician * Gustav Eisen, Swedish-American scientist * Hilda Eisen, Polish-born American businessperson, philanthropist, and Holocaust survivor * Jonathan Eisen, American biologist * Matthias Johann Eisen, Estonian folklorist *Michael Eisen, American biologist *Norm Eisen, American lawyer and diplomat * Percy A. Eisen, American architect *Rich Eisen, American television journalist * Sara Eisen, American television journalist *Stanley Bert "Paul Stanley Paul Stanley (born Stanley Bert Eisen; January 20, 1952) is an American musician who was the co-founder, frontman, rhythm gui ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anemia
Anemia (also spelt anaemia in British English) is a blood disorder in which the blood has a reduced ability to carry oxygen. This can be due to a lower than normal number of red blood cells, a reduction in the amount of hemoglobin available for oxygen transport, or abnormalities in hemoglobin that impair its function. The name is derived . When anemia comes on slowly, the symptoms are often vague, such as Fatigue, tiredness, weakness, shortness of breath, headaches, and a Exercise intolerance, reduced ability to exercise. When anemia is acute, symptoms may include confusion, lightheadedness, feeling like one is going to pass out, Syncope (medicine), loss of consciousness, and polydipsia, increased thirst. Anemia must be significant before a person becomes noticeably Pallor, pale. Additional symptoms may occur depending on the underlying cause. Anemia can be temporary or long term and can range from mild to severe. Anemia can be caused by blood loss, decreased red blood cel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins. Abundance It is estimated that approximately half of all proteins contain a metal. In another estimate, about one quarter to one third of all proteins are proposed to require metals to carry out their functions. Thus, metalloproteins have many different functions in cells, such as storage and transport of proteins, enzymes and signal transduction proteins, or infectious diseases. The abundance of metal binding proteins may be inherent to the amino acids that proteins use, as even artificial proteins without evolutionary history will readily bind metals. Most metals in the human body are bound to proteins. For instance, the relatively high concentration of iron in the human body ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin in the blood carries oxygen from the respiratory organs (lungs or gills) to the other tissues of the body, where it releases the oxygen to enable aerobic respiration which powers an animal's metabolism. A healthy human has 12to 20grams of hemoglobin in every 100mL of blood. Hemoglobin is a metalloprotein, a chromoprotein, and a globulin. In mammals, hemoglobin makes up about 96% of a red blood cell's dry matter, dry weight (excluding water), and around 35% of the total weight (including water). Hemoglobin has an oxygen-binding capacity of 1.34mL of O2 per gram, which increases the total blood oxygen capacity seventy-fold compared to dissolved oxygen in blood plasma alone. The mammalian hemoglobin molecule can bind and transport up to four ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic organism, anaerobic bacterium ''Clostridium pasteurianum''. Another redox protein, isolated from spinach chloroplasts, was termed "chloroplast ferredoxin". The chloroplast ferredoxin is involved in both cyclic and non-cyclic photophosphorylation reactions of photosynthesis. In non-cyclic photophosphorylation, ferredoxin is the last electron acceptor thus reducing the enzyme NADP+ reductase. It accepts electrons produced from sunlight-Electron excitation, excited chlorophyll and transfers them to the enzyme ferredoxin: NADP+ oxidoreductase . Ferredoxins are small proteins containing iron and sulfur atoms organized as iron–sulfur clusters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome
Cytochromes are redox-active proteins containing a heme, with a central iron (Fe) atom at its core, as a cofactor. They are involved in the electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of binding. Four varieties are recognized by the International Union of Biochemistry and Molecular Biology (IUBMB), cytochromes a, cytochromes b, cytochromes c and cytochrome d. Cytochrome function is linked to the reversible redox change from ferrous (Fe(II)) to the ferric (Fe(III)) oxidation state of the iron found in the heme core. In addition to the classification by the IUBMB into four cytochrome classes, several additional classifications such as cytochrome o and cytochrome P450 can be found in biochemical literature. History Cytochromes were initially described in 1884 by Charles Alexander MacMunn as respiratory pigments (myohematin or histohematin). In the 1920s, Keilin rediscovered these respiratory pigments and na ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
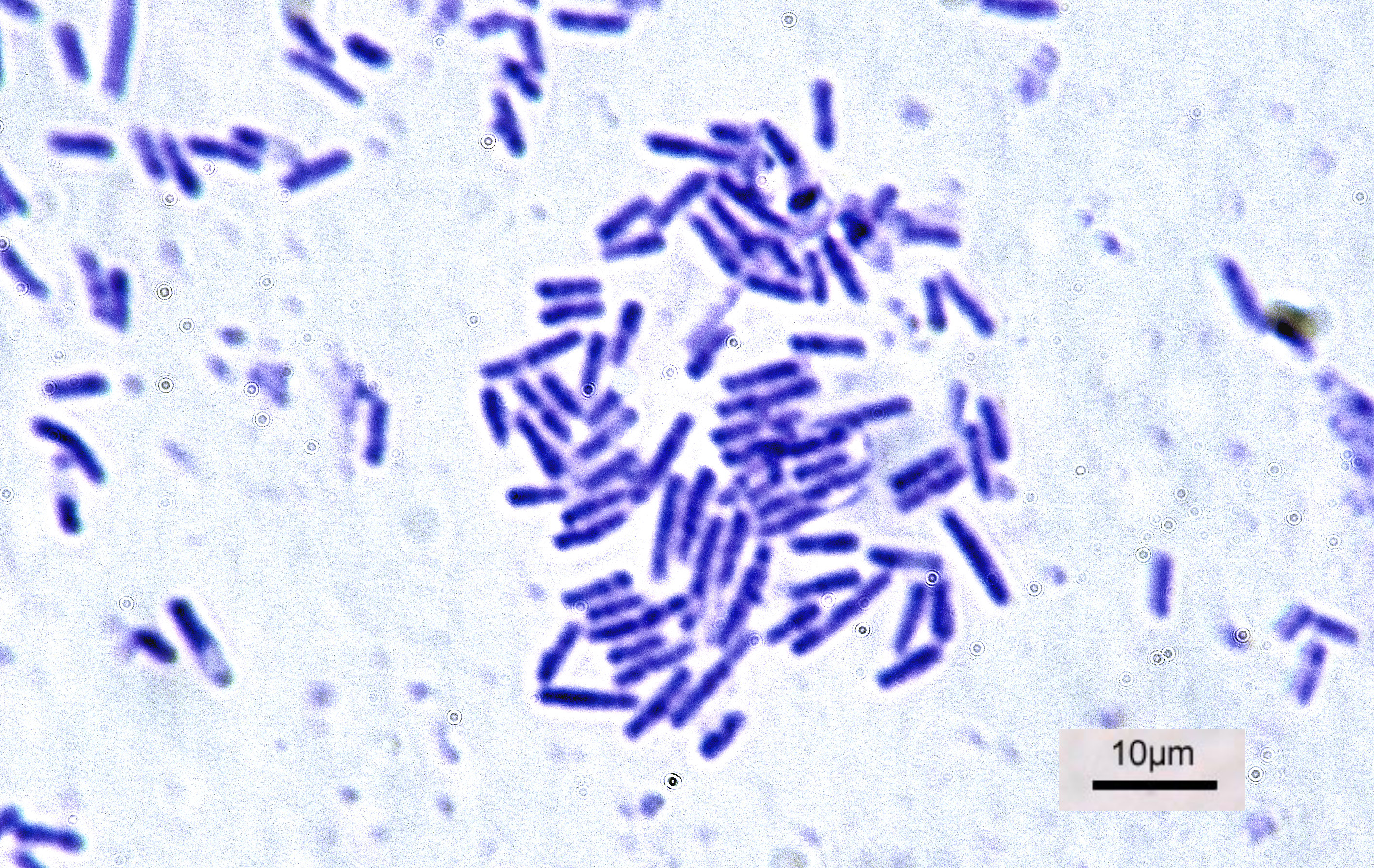
Bacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit the air, soil, water, Hot spring, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria play a vital role in many stages of the nutrient cycle by recycling nutrients and the nitrogen fixation, fixation of nitrogen from the Earth's atmosphere, atmosphere. The nutrient cycle includes the decomposition of cadaver, dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |