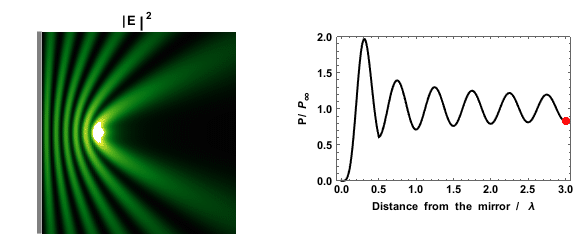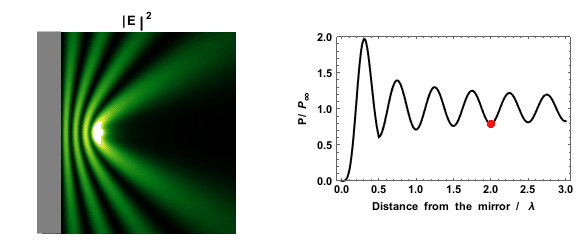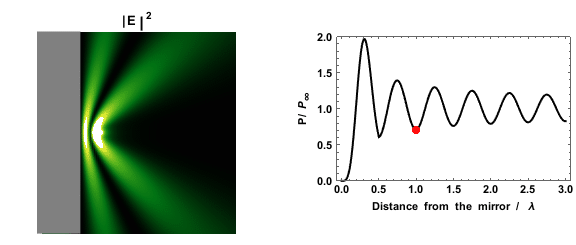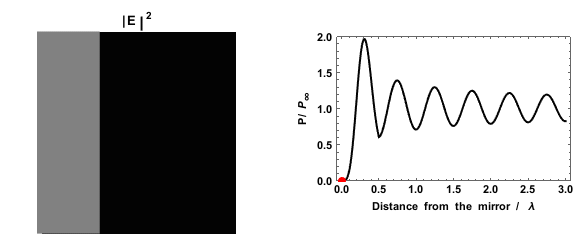
|
Electron Transfer
Electron transfer (ET) occurs when an electron relocates from an atom or molecule to another such chemical entity. ET is a mechanistic description of certain kinds of redox reactions involving transfer of electrons. Electrochemical processes are ET reaction. ET reactions are relevant to photosynthesis and respiration. ET reactions commonly involve transition metal complexes, In organic chemistry ET is a step in some commercial polymerization reactions. It is foundational to photoredox catalysis. Classes of electron transfer Inner-sphere electron transfer In inner-sphere ET, the two redox centers are covalently linked during the ET. This bridge can be permanent, in which case the electron transfer event is termed intramolecular electron transfer. More commonly, however, the covalent linkage is transitory, forming just prior to the ET and then disconnecting following the ET event. In such cases, the electron transfer is termed intermolecular electron transfer. A famous ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum ( spin) of a half-integer value, expressed in units of the reduced Planck constant, . Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery. The electrophore, invented by Johan Wilcke, was an early version of an electrode used to study static electricity. Anode and cathode in electrochemical cells Electrodes are an essential part of any battery. The first electrochemical battery made was devised by Alessandro Volta and was aptly named the Voltaic cell. This battery consisted of a stack of copper and zinc electrodes separated by brine-soaked paper disks. Due to fluctuation in the voltage provided by the voltaic cell it wasn't very practical. The first practical battery was invented in 1839 and named the Daniell cell after John Frederic Daniell. Still making use of the zinc–copper electrode combination. Since then many more batteries ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi's Golden Rule
In quantum physics, Fermi's golden rule is a formula that describes the transition rate (the probability of a transition per unit time) from one energy eigenstate of a quantum system to a group of energy eigenstates in a continuum, as a result of a weak perturbation. This transition rate is effectively independent of time (so long as the strength of the perturbation is independent of time) and is proportional to the strength of the coupling between the initial and final states of the system (described by the square of the matrix element of the perturbation) as well as the density of states. It is also applicable when the final state is discrete, i.e. it is not part of a continuum, if there is some decoherence in the process, like relaxation or collision of the atoms, or like noise in the perturbation, in which case the density of states is replaced by the reciprocal of the decoherence bandwidth. General Although the rule is named after Enrico Fermi, most of the work leading to it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alexander M
Alexander is a male given name. The most prominent bearer of the name is Alexander the Great, the king of the Ancient Greek kingdom of Macedonia who created one of the largest empires in ancient history. Variants listed here are Aleksandar, Aleksander and Aleksandr. Related names and diminutives include Iskandar, Alec, Alek, Alex, Alexandre, Aleks, Aleksa and Sander; feminine forms include Alexandra, Alexandria, and Sasha. Etymology The name ''Alexander'' originates from the (; 'defending men' or 'protector of men'). It is a compound of the verb (; 'to ward off, avert, defend') and the noun (, genitive: , ; meaning 'man'). It is an example of the widespread motif of Greek names expressing "battle-prowess", in this case the ability to withstand or push back an enemy battle line. The earliest attested form of the name, is the Mycenaean Greek feminine anthroponym , , (/Alexandra/), written in the Linear B syllabic script. Alaksandu, alternatively called ''Alakasandu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joshua Jortner
Joshua Jortner (Hebrew: יהושע יורטנר) (March 14, 1933) is an Israeli physical chemist. He is a professor emeritus at the School of Chemistry, The Sackler Faculty of Exact Sciences, Tel Aviv University in Tel Aviv, Israel. Birth and education Jortner was born on March 14, 1933, in Tarnów, Poland, to a Jewish family. He migrated with his parents to Palestine under the British Mandate during the Second World War in 1940. He received his Ph.D. from the Hebrew University of Jerusalem in 1960. Academic career After completing his Ph.D., Jortner became a lecturer in the Department of Physical Chemistry at the Hebrew University of Jerusalem from 1961 to 1963. From 1962 to 1964, he was a research associate at the University of Chicago. In 1964, he was appointed to a professorship in the Department of Chemistry at Tel Aviv University and was its first chairman. From 1966 to 1972, he was deputy rector, acting rector and vice president of Tel Aviv University. Since 1973, he ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanical
Quantum mechanics is a fundamental theory in physics that provides a description of the physical properties of nature at the scale of atoms and subatomic particles. It is the foundation of all quantum physics including quantum chemistry, quantum field theory, quantum technology, and quantum information science. Classical physics, the collection of theories that existed before the advent of quantum mechanics, describes many aspects of nature at an ordinary ( macroscopic) scale, but is not sufficient for describing them at small (atomic and subatomic) scales. Most theories in classical physics can be derived from quantum mechanics as an approximation valid at large (macroscopic) scale. Quantum mechanics differs from classical physics in that energy, momentum, angular momentum, and other quantities of a bound system are restricted to discrete values ( quantization); objects have characteristics of both particles and waves (wave–particle duality); and there are limits to ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noel Hush
Noel Sydney Hush (15 December 1924 – 20 March 2019) was an Australian chemist at the University of Sydney. Career Hush was born in Sydney on 15 December 1924 and obtained his BSc hons (1945) and MSc (1948) at the University of Sydney, where he worked as a research fellow in the Department of Chemistry (1945–49). He then accepted an invitation from M. G. Evans FRS to work in England as an assistant lecturer at the University of Manchester (1950–54) in the department created by Michael Polanyi. He was subsequently lecturer and then reader in the Department of Chemistry, University of Bristol (1955–71). He returned to Australia in 1971 to found the Department of Theoretical Chemistry at the University of Sydney, the first such department in Australia. In 1989 he became a full-time research-only emeritus professor. He has held numerous prestigious visiting scientist positions at universities in Australia, the UK, and the US. Adiabatic electron transfer A unifying theme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inner-sphere Electron Transfer
Inner sphere electron transfer (IS ET) or bonded electron transfer is a redox chemical reaction that proceeds via a covalent linkage—a strong electronic interaction—between the oxidant and the reductant reactants. In inner sphere electron transfer, a ligand bridges the two metal redox centers during the electron transfer event. Inner sphere reactions are inhibited by large ligands, which prevent the formation of the crucial bridged intermediate. Thus, inner sphere ET is rare in biological systems, where redox sites are often shielded by bulky proteins. Inner sphere ET is usually used to describe reactions involving transition metal complexes and most of this article is written from this perspective. However, redox centers can consist of organic groups rather than metal centers. The bridging ligand could be virtually any entity that can convey electrons. Typically, such a ligand has more than one lone electron pair, such that it can serve as an electron donor to both the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition-state Theory
In chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes. TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ''H''‡, also written Δ‡''H''ɵ), the standard entropy of activation (Δ''S''‡ or Δ‡''S''ɵ), and the standard Gibbs energy of activation (Δ''G''‡ or Δ‡''G''ɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest ''at the transition state''; Δ''H''‡ is the difference between t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Outer-sphere Electron Transfer
Outer sphere refers to an electron transfer (ET) event that occurs between chemical species that remain separate and intact before, during, and after the ET event. In contrast, for inner sphere electron transfer the participating redox sites undergoing ET become connected by a chemical bridge. Because the ET in outer sphere electron transfer occurs between two non-connected species, the electron is forced to move through space from one redox center to the other. Marcus theory The main theory that describes the rates of outer sphere electron transfer was developed by Rudolph A. Marcus in the 1950s. A major aspect of Marcus theory is the dependence of the electron transfer rate on the thermodynamic driving force (difference in the redox potentials of the electron-exchanging sites). For most reactions, the rates increase with increased driving force. A second aspect is that the rate of outer sphere electron-transfer depends inversely on the "reorganizational energy." Reorganization ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rudolph A
Rudolph or Rudolf may refer to: People * Rudolph (name), the given name including a list of people with the name Religious figures * Rudolf of Fulda (died 865), 9th century monk, writer and theologian * Rudolf von Habsburg-Lothringen (1788–1831), Archbishop of Olomouc and member of the House of Habsburg-Lorraine Royalty and nobility *Rudolph I (other) * Rudolph II (other) * Rudolph III (other) * Rudolph of France (died 936) * Rudolph I of Germany (1218–1291) * Rudolf II, Holy Roman Emperor (1552–1612) * Rudolph, Prince of Anhalt-Zerbst (1576–1621) * Rudolf, Crown Prince of Austria (1858–1889), son and heir of Emperor Franz Joseph I of Austria and Empress Elisabeth of Austria (died at Mayerling) Places * Rudolph Glacier, Antarctica * Rudolph, South Dakota, US * Rudolph, Wisconsin, US, a village * Rudolph (town), Wisconsin, adjacent to the village * Rudolf Island, northernmost island of Europe * Lake Rudolf, now Lake Turkana, in Ken ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-sulfur Cluster
Iron–sulfur proteins (or iron–sulphur proteins in British spelling) are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are vulnera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |