|
EF Hand
The EF hand is a helix–loop–helix structural domain or ''motif'' found in a large family of calcium-binding proteins. The EF-hand motif contains a helix–loop–helix topology, much like the spread thumb and forefinger of the human hand, in which the Ca2+ ions are coordinated by ligands within the loop. The motif takes its name from traditional nomenclature used in describing the protein parvalbumin, which contains three such motifs and is probably involved in muscle relaxation via its calcium-binding activity. The EF-hand consists of two alpha helices linked by a short loop region (usually about 12 amino acids) that usually binds calcium ions. EF-hands also appear in each structural domain of the signaling protein calmodulin and in the muscle protein troponin-C. Calcium ion binding site The calcium ion is coordinated in a pentagonal bipyramidal configuration. The six residues involved in the binding are in positions 1, 3, 5, 7, 9 and 12; these residues are denoted by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paramecium
'' ''Paramecium'' ( , ; also spelled ''Paramoecium'') is a genus of eukaryotic, unicellular ciliates, commonly studied as a representative of the ciliate group. ''Paramecia'' are widespread in freshwater, brackish, and marine environments and are often very abundant in stagnant basins and ponds. Because some species are readily cultivated and easily induced to conjugate and divide, it has been widely used in classrooms and laboratories to study biological processes. Its usefulness as a model organism has caused one ciliate researcher to characterize it as the "white rat" of the phylum Ciliophora. Historical background ''Paramecia'' were among the first ciliates to be seen by microscopists, in the late 17th century. They were probably known to the Dutch pioneer of protozoology, Antonie van Leeuwenhoek, and were clearly described by his contemporary Christiaan Huygens in a letter of 1678. The earliest known illustration of a Paramecium was published anonymously in Philosophi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartate
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. Like all other amino acids, it contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, −COO−. It is a non-essential amino acid in humans, meaning the body can synthesize it as needed. It is encoded by the codons GAU and GAC. D-Aspartate is one of two D-amino acids commonly found in mammals. .html" ;"title="/sup>">/sup> In proteins aspartate sidechains are often hydrogen bonded to form asx turns or asx motifs, which frequently occur at th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphingomonas
''Sphingomonas'' was defined in 1990 as a group of Gram-negative, rod-shaped, chemoheterotrophic, strictly aerobic bacteria. They possess ubiquinone 10 as their major respiratory quinone, contain glycosphingolipids (GSLs), specifically ceramide, instead of lipopolysaccharide (LPS) in their cell envelopes, and typically produce yellow-pigmented colonies. The GSL serves to protect the bacteria from antibacterial substances. Unlike most Gram-negative bacteria, ''Sphingomonas'' carries endotoxins and has a hydrophobic surface characterized by the short nature of the GSL's carbohydrate portion. By 2001, the genus included more than 20 species that were quite diverse in terms of their phylogenetic, ecological, and physiological properties. As a result, ''Sphingomonas'' was subdivided into different genera: ''Sphingomonas'', ''Sphingobium'', '' Novosphingobium'', '' Sphingosinicella'', and '' Sphingopyxis''. These genera are commonly referred to collectively as sphingomonads. Distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salmonella Typhimurium
''Salmonella enterica'' subsp. ''enterica'' is a subspecies of ''Salmonella enterica'', the rod-shaped, flagellated, aerobic, Gram-negative bacterium. Many of the pathogenic serovars of the ''S. enterica'' species are in this subspecies, including that responsible for typhoid. Serovars ''S. enterica'' subsp. ''enterica'' contains a large number of serovars which can infect a broad range of vertebrate hosts. The individual members range from being highly host-adapted (only able to infect a narrow range of species) to displaying a broad host range. A number of techniques are currently used to differentiate between serotypes. These include looking for the presence or absence of antigens, phage typing, molecular fingerprinting and biotyping, where serovars are differentiated by which nutrients they are able to ferment. A possible factor in determining the host range of particular serovars is phage-mediated acquisition of a small number of genetic elements that enable infection of a pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacillus Subtilis
''Bacillus subtilis'', known also as the hay bacillus or grass bacillus, is a Gram-positive, catalase-positive bacterium, found in soil and the gastrointestinal tract of ruminants, humans and marine sponges. As a member of the genus ''Bacillus'', ''B. subtilis'' is rod-shaped, and can form a tough, protective endospore, allowing it to tolerate extreme environmental conditions. ''B. subtilis'' has historically been classified as an obligate aerobe, though evidence exists that it is a facultative anaerobe. ''B. subtilis'' is considered the best studied Gram-positive bacterium and a model organism to study bacterial chromosome replication and cell differentiation. It is one of the bacterial champions in secreted enzyme production and used on an industrial scale by biotechnology companies. Description ''Bacillus subtilis'' is a Gram-positive bacterium, rod-shaped and catalase-positive. It was originally named ''Vibrio subtilis'' by Christian Gottfried Ehrenberg, and renamed ''Bacil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calbindin D9k
Calbindins are three different calcium-binding proteins: calbindin, calretinin and S100G. They were originally described as vitamin D-dependent calcium-binding proteins in the intestine and kidney in the chick and mammals. They are now classified in different subfamilies as they differ in the number of Ca2+ binding EF hands. Calbindin 1 Calbindin 1 or simply calbindin was first shown to be present in the intestine in birds and then found in the mammalian kidney. It is also expressed in a number of neuronal and endocrine cells, particularly in the cerebellum. It is a 28 kDa protein encoded in humans by the ''CALB1'' gene. Calbindin contains 4 active calcium-binding domains, and 2 modified domains that have lost their calcium-binding capacity. Calbindin acts as a calcium buffer and calcium sensor and can hold four Ca2+ in the EF-hands of loops EF1, EF3, EF4 and EF5. The structure of rat calbindin was originally solved by nuclear magnetic resonance and was one of the largest p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calreticulin
Calreticulin also known as calregulin, CRP55, CaBP3, calsequestrin-like protein, and endoplasmic reticulum resident protein 60 (ERp60) is a protein that in humans is encoded by the ''CALR'' gene. Calreticulin is a multifunctional soluble protein that binds Ca2+ ions (a second messenger in signal transduction), rendering it inactive. The Ca2+ is bound with low affinity, but high capacity, and can be released on a signal (see inositol trisphosphate). Calreticulin is located in storage compartments associated with the endoplasmic reticulum and is considered an ER resident protein. The term "Mobilferrin" is considered to be the same as calreticulin by some sources. Function Calreticulin binds to misfolded proteins and prevents them from being exported from the endoplasmic reticulum to the Golgi apparatus. A similar quality-control molecular chaperone, calnexin, performs the same service for soluble proteins as does calreticulin, however it is a membrane-bound protein. Bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
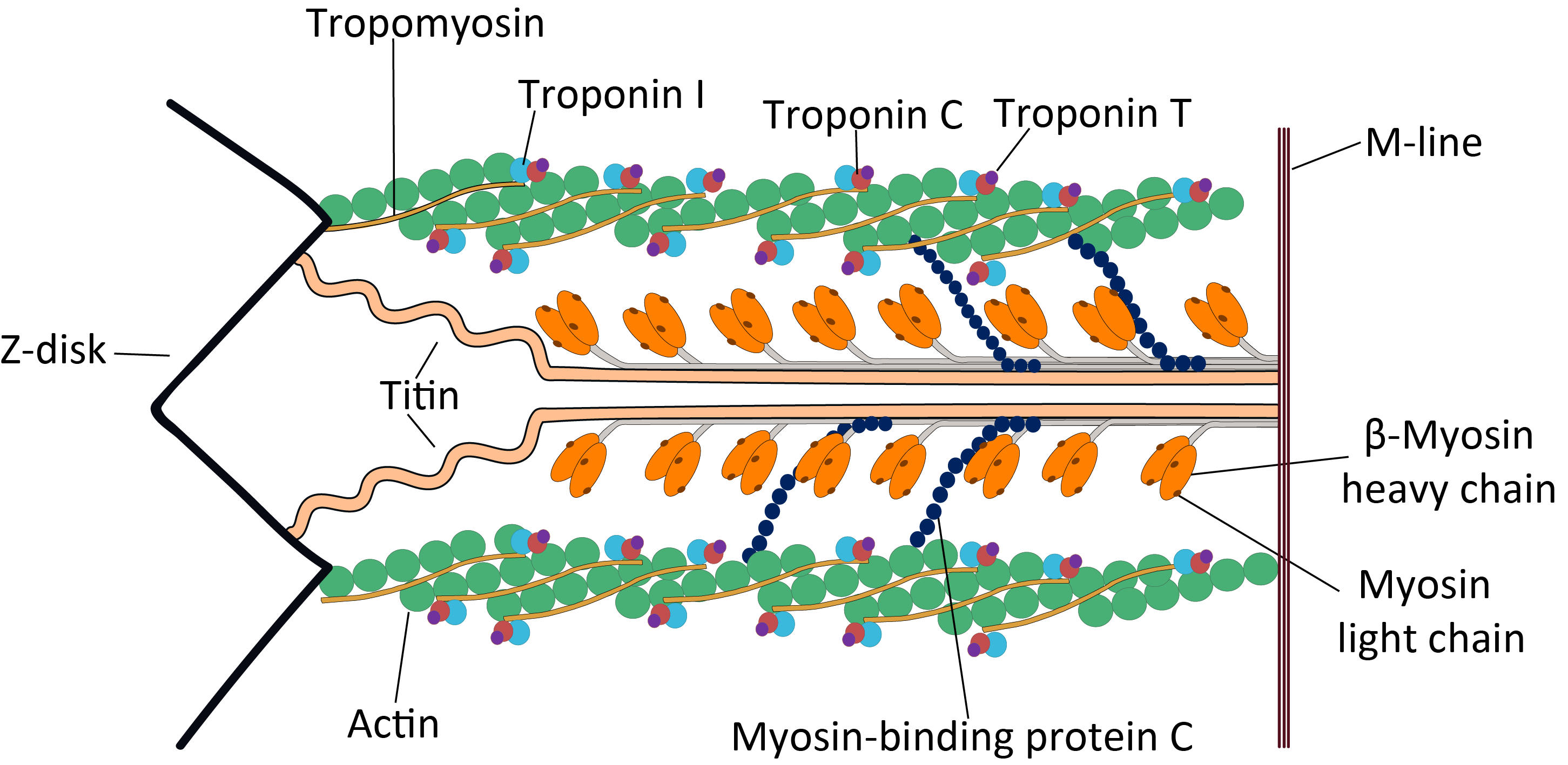
Troponin C
Troponin C is a protein which is part of the troponin complex. It contains four calcium-binding EF hands, although different isoforms may have fewer than four functional calcium-binding subdomains. It is a component of thin filaments, along with actin and tropomyosin. It contains an N lobe and a C lobe. The C lobe serves a structural purpose and binds to the N domain of troponin I (TnI). The C lobe can bind either Ca2+ or Mg2+. The N lobe, which binds only Ca2+, is the regulatory lobe and binds to the C domain of troponin I after calcium binding. Isoforms The tissue specific subtypes are: * Slow troponin C, TNNC1 (3p21.1 ) * Fast troponin C, TNNC2 (20q12-q13.11, ) Mutations Point mutations can occur in troponin C inducing alterations to Ca2+ and Mg2+ binding and protein structure, leading to abnormalities in muscle contraction. In cardiac muscle, they are related to dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM). These known point mutations are: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic Core
The hydrophobic effect is the observed tendency of nonpolar substances to aggregate in an aqueous solution and exclude water molecules. The word hydrophobic literally means "water-fearing", and it describes the segregation of water and nonpolar substances, which maximizes hydrogen bonding between molecules of water and minimizes the area of contact between water and nonpolar molecules. In terms of thermodynamics, the hydrophobic effect is the free energy change of water surrounding a solute. A positive free energy change of the surrounding solvent indicates hydrophobicity, whereas a negative free energy change implies hydrophilicity. The hydrophobic effect is responsible for the separation of a mixture of oil and water into its two components. It is also responsible for effects related to biology, including: cell membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Hence the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. Hydrophobic is often used interchangeably with lipophilic, "fat-loving". However, the two terms are not synonymous. While hydrophobic substances are usually lipophilic, there are exceptions, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |