|
Electron-withdrawing Group
An electron-withdrawing group (EWG) is a Functional group, group or atom that has the ability to draw electron density toward itself and away from other adjacent atoms. This electron density transfer is often achieved by resonance or inductive effects. Electron-withdrawing groups have significant impacts on fundamental chemical processes such as acid-base reactions, redox potentials, and substitution reactions. Consequences of EWGs Effects on Brønsted–Lowry acidity Electron-withdrawing groups exert an "Inductive effect, inductive" or "electron-pulling" effect on covalent bonds. The strength of the electron-withdrawing group is inversely proportional to the Acid dissociation constant, pKa of the carboxylic acid. : The inductive effect is cumulative: trichloroacetic acid is 1000× stronger than chloroacetic acid. : :The impact of the EWG on pKa decreases with distances from the carboxylic group. For benzoic acids, the effect is quantified by the Hammett equation: :\log ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Functional Group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis. The Reactivity (chemistry), reactivity of a functional group can be modified by other functional groups nearby. Functional group interconversion can be used in retrosynthetic analysis to plan organic synthesis. A functional group is a group of atoms in a molecule with distinctive Chemical property, chemical properties, regardless of the other atoms in the molecule. The atoms in a functional group are linked to each other and to the rest of the molecule by covalent bonds. For repeating units of polymers, functional groups attach to their Chemical polarity, nonp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tolman Electronic Parameter
The Tolman electronic parameter (TEP) is a measure of the electron donating or withdrawing ability of a ligand. It is determined by measuring the frequency of the A1 C-O vibrational mode (ν(CO)) of a (pseudo)-C3v Molecular symmetry, symmetric complex, [LNi(CO)3] by infrared spectroscopy, where L is the ligand of interest. [LNi(CO)3] was chosen as the model compound because such complexes are readily prepared from tetracarbonylnickel(0). The shift in ν(CO) is used to infer the electronic properties of a ligand, which can aid in understanding its behavior in other complexes. The analysis was introduced by Chadwick A. Tolman. : The A1 carbonyl band is rarely obscured by other bands in the analyte's infrared spectrum. Carbonyl is a small ligand so steric factors do not complicate the analysis. Upon coordination of CO to a metal, ν(CO) typically decreases from 2143 cm−1 of free CO. This shift can be explained by Pi backbonding, π backbonding: the metal forms a π bond with the car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
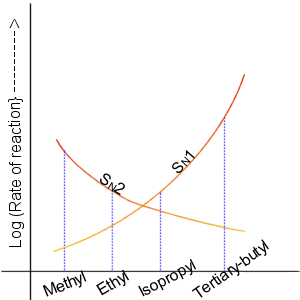
Nucleophilic Substitution Reaction
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :OH- + ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic ring, aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, alkylation Friedel–Crafts reaction and acylation Friedel–Crafts reaction. Illustrative reactions The most widely practised example of this reaction is the ethylation of benzene. :: Approximately 24,700,000 tons were produced in 1999. (After dehydrogenation and polymerization, the commodity plastic polystyrene is produced.) In this process, acids are used as catalyst to generate the incipient carbocation. Many other electrophilic reactions of benzene are conducted, although on a much smaller scale; they are valuable routes to key intermediates. The nitration of benzene is achieved via the action of the nitronium ion as the electrophile. The Aromatic sulfonation, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Ortho Meta Para
Ortho- is a Greek prefix meaning “straight”, “upright”, “right” or “correct”. Ortho may refer to: * Ortho, Belgium, a village in the Belgian province of Luxembourg Science * List of commonly used taxonomic affixes (ortho-) * Arene substitution patterns, two substituents that occupy adjacent positions on an aromatic ring * Chlordane, an organochlorine compound that was used as a pesticide Mathematics * Orthogonal, a synonym for perpendicular * Orthonormal, the property that a collection of vectors are mutually perpendicular and each of unit magnitude * Orthodrome, a synonym for great circle, a geodesic on the sphere * Orthographic projection, a parallel projection onto a perpendicular plane Medicine * Orthomyxovirus, a family of viruses to which influenza belongs * Orthodontics, a specialty of dentistry concerned with the study and treatment of malocclusions * Orthopedic, the study of the musculoskeletal system * Ortho-DOT, a psychedelic drug * Ortho-cept and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphorus Trifluoride
Phosphorus trifluoride (formula P F3), is a colorless and odorless gas. It is highly toxic and reacts slowly with water. Its main use is as a ligand in metal complexes. As a ligand, it parallels carbon monoxide in metal carbonyls, and indeed its toxicity is due to its binding with the iron in blood hemoglobin in a similar way to carbon monoxide. Physical properties Phosphorus trifluoride has an F−P−F bond angle of approximately 96.3°. Gaseous PF3 has a standard enthalpy of formation of −945 kJ/mol (−226 kcal/ mol). The phosphorus atom has a nuclear magnetic resonance chemical shift of 97 ppm (downfield of H3PO4). Properties Phosphorus trifluoride hydrolyzes especially at high pH, but it is less hydrolytically sensitive than phosphorus trichloride. It does not attack glass except at high temperatures, and anhydrous potassium hydroxide may be used to dry it with little loss. With hot metals, phosphides and fluorides are formed. With Lewis base ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Phosphorus Trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride. History Phosphorus trichloride was first prepared in 1808 by the French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard by heating calomel (Hg2Cl2) with phosphorus. Later during the same year, the English chemist Humphry Davy produced phosphorus trichloride by burning phosphorus in chlorine gas. Preparation World production exceeds one-third of a million tonnes. Phosphorus trichloride is prepared industrially by the reaction of chlorine with white phosphorus, using phosphorus trichloride as the solvent. In this continuous process PCl3 is removed as it is formed in order to avoid the formation of PCl5. :P4 + 6 Cl2 → 4 PCl3 Structure and spectroscopy It has a t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Triethyl Phosphite
Triethyl phosphite (TEP) is an organophosphorus compound, specifically a phosphite ester, with the formula P(OCH2CH3)3, often abbreviated P(OEt)3. It is a colorless, malodorous liquid. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three ethoxide groups. Its 31P NMR spectrum features a signal at around +139 ppm vs phosphoric acid standard. Triethylphosphite is prepared by treating phosphorus trichloride with ethanol in the presence of a base, typically a tertiary amine: :PCl3 + 3 EtOH + 3 R3N → P(OEt)3 + 3 R3NH + 3 Cl− In the absence of the base, the reaction of ethanol and phosphorus trichloride affords diethylphosphite ((EtO)2P(O)H). Of the many related compounds can be prepared similarly, triisopropyl phosphite is an example (b.p. 43.5 °C/1.0 mm; CAS# 116-17-6). Reactions Triethyl phosphite can react with electrophiles in a Michaelis–Arbuzov rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tris(4-fluorophenyl)phosphine
Tris, or tris(hydroxymethyl)aminomethane, or known during medical use as tromethamine or THAM, is an organic compound with the formula (HOCH2)3CNH2. It is extensively used in biochemistry and molecular biology as a component of buffer solutions such as in TAE and TBE buffers, especially for solutions of nucleic acids. It contains a primary amine and thus undergoes the reactions associated with typical amines, e.g., condensations with aldehydes. Tris also complexes with metal ions in solution. In medicine, tromethamine is occasionally used as a drug, given in intensive care for its properties as a buffer for the treatment of severe metabolic acidosis in specific circumstances. Some medications are formulated as the "tromethamine salt" including Hemabate (carboprost as trometamol salt), and "ketorolac trometamol". In 2023 a strain of ''Pseudomonas hunanensis'' was found to be able to degrade TRIS buffer. Since Tris' pKa is more strongly temperature dependent, its use is not recom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the thre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tris(4-methoxyphenyl)phosphine
Tris(4-methoxyphenyl)phosphine is the organophosphorus compound with the formula (CH3OC6H4)3P. Several isomers of this formula are known, but the symmetrical derivative with methoxy groups in the 4-position is most studied. The compound is used as a ligand in organometallic chemistry and homogeneous catalysis.{{cite journal , doi=10.1039/j19680003133, title=Hydroformylation of alkenes by use of rhodium complex catalysts, year=1968, last1=Evans, first1=D., last2=Osborn, first2=J. A., last3=Wilkinson, first3=G., journal=Journal of the Chemical Society A: Inorganic, Physical, Theoretical, page=3133 Related ligands *Triphenylphosphine Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a l ... * 2-(Diphenylphosphino)anisole References Tertiary phosphines 4-Methoxyphenyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Trimethylphosphine
Trimethylphosphine is an organophosphorus compound with the formula P(CH3)3, commonly abbreviated as PMe3. This colorless liquid has a strongly unpleasant odor, characteristic of alkylphosphines. The compound is a common ligand in coordination chemistry. Structure and bonding It is a pyramidal molecule with approximate ''C''3''v'' point group, symmetry. The C–P–C bond angles are approximately 98.6°. The C–P–C bond angles are consistent with the notion that phosphorus predominantly uses the 3p orbitals for forming bonds and that there is little sp hybridization of the phosphorus atom. The latter is a common feature of the chemistry of phosphorus. As a result, the lone pair of trimethylphosphine has predominantly s-character as is the case for phosphine, PH3. PMe3 can be prepared by the treatment of triphenyl phosphite with methylmagnesium chloride: : 3 CH3MgCl + P(OC6H5)3 → P(CH3)3 + 3 C6H5OMgCl The synthesis is conducted in dibutyl ether, from which the more volatil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |