|
Effervescence
Effervescence is the escape of gas from an aqueous solution and the foaming or fizzing that results from that release. The word effervescence is derived from the Latin verb ''fervere'' (to boil), preceded by the adverb ''ex''. It has the same linguistic root as the word fermentation. Effervescence can also be observed when opening a bottle of champagne, beer or carbonated beverages such as some carbonated soft drinks. The visible bubbles are produced by the escape from solution of the dissolved gas (which itself is not visible while dissolved in the liquid). In beverages Although CO2 is most common for beverages, nitrogen gas is sometimes deliberately added to certain beers. The smaller bubble size creates a smoother beer head. Due to the poor solubility of nitrogen in beer, kegs or widgets are used for this. Chemistry In the laboratory, a common example of effervescence is seen if hydrochloric acid is added to a block of limestone. If a few pieces of marble or an an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitation (chemistry)
In an aqueous solution, precipitation is the "sedimentation of a solid material (a precipitate) from a liquid solution". The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant. The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the supernate or supernatant. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases (e.g. metallurgy and alloys) when solid impurities segregation (materials science), segregate from a solid phase. Supersaturation The precipitation of a compound may occur when its concentration exceeds its solubility. This can be due to temperature changes, solvent evaporation, or by mixing solvents. Precipitation occurs more rapidly from a strongly supersaturated solution. The formation of a pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Test Tube
A test tube, also known as a culture tube or sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom. Test tubes are usually placed in special-purpose test tube rack, racks. Types and usage Chemistry Test tubes intended for general chemical work are usually made of glass, for its relative resistance to heat. Tubes made from expansion-resistant glasses, mostly borosilicate glass or fused quartz, can withstand high temperatures up to several hundred degrees Celsius. Chemistry tubes are available in a multitude of lengths and widths, typically from 10 to 20 mm wide and 50 to 200 mm long. The top often features a flared lip to aid pouring out the contents. A chemistry test tube typically has a flat bottom, a round bottom, or a conical bottom. Some test tubes are made to accept a ground glass joint, ground glass stopper or a screw cap. They are often provided w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Effervescent Tablet
Effervescent or carbon tablets are tablets which are designed to dissolve in water and release carbon dioxide. The carbon dioxide is generated by a reaction of a compound containing bicarbonate, such as sodium bicarbonate or magnesium bicarbonate, with an acid such as citric acid or tartaric acid. Both compounds are present in the tablet in powder form and start reacting as soon as they dissolve in water. Effervescent tablets are made by Tableting, compression of ingredients in the form of powders into a dense mass, which is packaged in blister pack, or with a hermetic seal, hermetically sealed package with incorporated desiccant in the cap. To use them, they are dropped into water to make a Solution (chemistry), solution. The powdered ingredients are also packaged and sold as effervescent powders or may be granulated and sold as effervescent granules. Generally powdered ingredients are first granularized before being made into tablets. Effervescent medicinal beverages date bac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonation
Carbonation is the chemical reaction of carbon dioxide to give carbonates, bicarbonates, and carbonic acid. In chemistry, the term is sometimes used in place of carboxylation, which refers to the formation of carboxylic acids. In inorganic chemistry and geology, carbonation is common. Metal hydroxides (MOH) and metal oxides (M'O) react with CO2 to give bicarbonates and carbonates: :MOH + CO2 → M(HCO3) :M'O + CO2 → M'CO3 Selected carbonations Carbonic anhydrase In mammalian physiology, transport of carbon dioxide to the lungs involves a carbonation reaction catalyzed by the enzyme carbonic anhydrase. In the absence of such catalysts, carbon dioxide cannot be expelled sufficient rate to support metabolic needs. The enzyme harbors a zinc aquo complex, which captures carbon dioxide to give a zinc bicarbonate: : Behavior of concrete In reinforced concrete, the chemical reaction between carbon dioxide in the air and calcium hydroxide and hydrated calcium silicate in t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
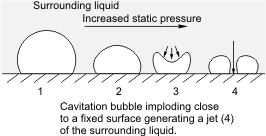
Cavitation
Cavitation in fluid mechanics and engineering normally is the phenomenon in which the static pressure of a liquid reduces to below the liquid's vapor pressure, leading to the formation of small vapor-filled cavities in the liquid. When subjected to higher pressure, these cavities, called "bubbles" or "voids", collapse and can generate shock waves that may damage machinery. These shock waves are strong when they are very close to the imploded bubble, but rapidly weaken as they propagate away from the implosion. Cavitation is a significant cause of wear in some engineering contexts. Collapsing voids that implode near to a metal surface cause cyclic stress through repeated implosion. This results in surface fatigue of the metal, causing a type of wear also called "cavitation". The most common examples of this kind of wear are to pump impellers, and bends where a sudden change in the direction of liquid occurs. Cavitation is usually divided into two classes of behavior. ''In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decompression (physics)
In mechanics, compression is the application of balanced inward ("pushing") forces to different points on a material or Structural system, structure, that is, forces with no Net force, net sum or torque directed so as to reduce its size in one or more directions.Ferdinand Pierre Beer, Elwood Russell Johnston, John T. DeWolf (1992), "Mechanics of Materials". (Book) McGraw-Hill Professional, It is contrasted with tension (physics), tension or traction, the application of balanced outward ("pulling") forces; and with shear stress, shearing forces, directed so as to displace layers of the material parallel to each other. The compressive strength of materials and structures is an important engineering consideration. In uniaxial compression, the forces are directed along one direction only, so that they act towards decreasing the object's length along that direction. The compressive forces may also be applied in multiple directions; for example inwards along the edges of a plate or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonic Acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion of carbon dioxide and carbonic acid is related to the breathing cycle of animals and the acidification of natural waters. In biochemistry and physiology, the name "carbonic acid" is sometimes applied to aqueous solutions of carbon dioxide. These chemical species play an important role in the bicarbonate buffer system, used to maintain acid–base homeostasis. Terminology in biochemical literature In chemistry, the term "carbonic acid" strictly refers to the chemical compound with the formula . Some biochemistry literature effaces the distinction between carbonic acid and carbon dioxide dissolved in extracellular fluid. In physiology, carbon dioxide excreted by the lungs may be called ''volatile acid'' or ''respiratory acid''. Anh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bung
A stopper, bung, or cork is a cylindrical or conical closure used to seal a container, such as a bottle, tube, or barrel. Description Unlike a lid or bottle cap, which encloses a container from the outside without displacing the inner volume, a bung is partially or wholly inserted inside the container to act as a seal. A bung can be defined as "a plug or closure used to close an opening in a drum or barrel. It is called a plug when referring to a steel drum closure." A glass stopper is often called a "ground glass joint" (or "joint taper"), and a cork stopper is called simply a "cork". Stoppers used for wine bottles are referred to as "corks", even when made from another material. A common every-day example of a stopper is the cork of a wine bottle. When used to seal the bungholes of barrels, the stopper is called a bung. Other bungs, particularly those used in chemical barrels, may be made of metal and be screwed into place via threading. Ground glass joint Gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Soda Bubbles Macro
Soda or SODA may refer to: *Soft drink, a sweetened, carbonated, and usually flavored drink Chemistry * Some chemical compounds containing sodium ** Sodium carbonate, washing soda or soda ash ** Sodium bicarbonate, baking soda ** Sodium hydroxide, caustic soda ** Sodium oxide, an alkali metal oxide * Soda glass, a common glass made with sodium carbonate or sodium oxide * Soda lake, an alternate generic name for a salt lake, with high concentration of sodium carbonates * Soda lime, a mixture of sodium, calcium, and potassium hydroxides * Soda pulping, a process for paper production using sodium compounds Computing * SODA (operating system) * Service-oriented development of applications * Service-oriented device architecture, to enable devices to be connected to a service-oriented architecture * Soda PDF, a family of applications used on .pdf files * Symposium on Discrete Algorithms, an annual academic conference in computer science Entertainment * '' Czech Soda'' (Česká ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aqueous Solution
An aqueous solution is a solution in which the solvent is water. It is mostly shown in chemical equations by appending (aq) to the relevant chemical formula. For example, a solution of table salt, also known as sodium chloride (NaCl), in water would be represented as . The word ''aqueous'' (which comes from ''aqua'') means pertaining to, related to, similar to, or dissolved in, water. As water is an excellent solvent and is also naturally abundant, it is a ubiquitous solvent in chemistry. Since water is frequently used as the solvent in experiments, the word solution refers to an aqueous solution, unless the solvent is specified. A ''non-aqueous solution'' is a solution in which the solvent is a liquid, but is not water. Characteristics Substances that are ''hydrophobic'' ('water-fearing') do not dissolve well in water, whereas those that are '' hydrophilic'' ('water-friendly') do. An example of a hydrophilic substance is sodium chloride. In an aqueous solution the hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |