|
Dexter Electron Transfer
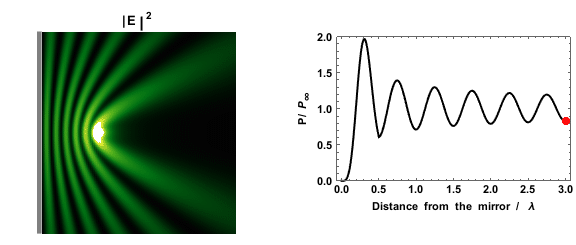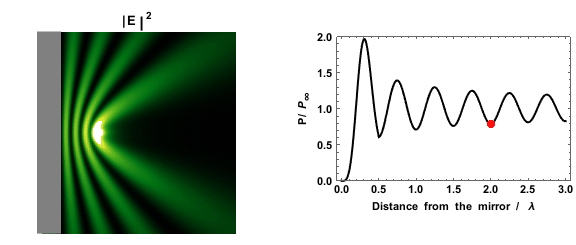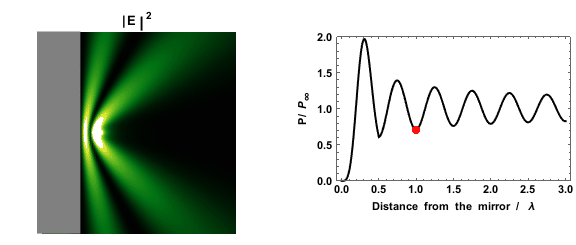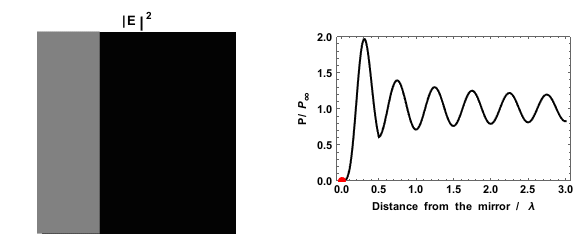
Dexter electron transfer (also called Dexter electron exchange and Dexter energy transfer) is a fluorescence quenching (fluorescence), quenching mechanism in which an Excited state, excited electron is transferred from one molecule (a Electron donor, donor) to a second molecule (an Electron acceptor, acceptor) via a Non-radiative recombination, non radiative path. This process requires a Wave function, wavefunction overlap between the donor and acceptor, which means it can only occur at short distances; typically within 10 Å (1 nm). The excited state may be exchanged in a single step, or in two separate charge exchange steps. History This short range energy transfer process was first theoretically proposed by D. L. Dexter in 1953. Rate expression The Dexter energy transfer rate, k_, is indicated by the formula: :k_ = K J' \mathrm\left [ \frac \right ] where r is the separation of the donor from the acceptor, L is the sum of the Van der Waals radius, Van der Waals radii of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dexter Electron (energy) Transfer
Dexter may refer to: People * Dexter (given name) * Dexter (surname) * Dexter (singer), Brazilian rapper Marcos Fernandes de Omena (born 1973) * Famous Dex, also known as Dexter, American rapper Dexter Tiewon Gore Jr. (born 1993) Places United States * Dexter, Georgia, a town * Dexter, Illinois, an unincorporated community * Dexter, Indiana, an unincorporated community * Dexter, Iowa, a city * Dexter Township, Cowley County, Kansas ** Dexter, Kansas, a city * Dexter, Kentucky, an unincorporated community * Dexter, Maine, a town ** Dexter (CDP), Maine, a census-designated place ** Dexter Regional Airport * Dexter Township, Michigan * Dexter, Michigan, a city located near, though distinct from, Dexter Township * Dexter Township, Mower County, Minnesota ** Dexter, Minnesota, a city * Dexter, Missouri, a city * Dexter, New Mexico, a town * Dexter, New York, a village ** Dexter Marsh * Dexter City, Ohio, a village * Dexter, Oregon, an unincorporated community in Lane County * Dexter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wave Function
In quantum physics, a wave function (or wavefunction) is a mathematical description of the quantum state of an isolated quantum system. The most common symbols for a wave function are the Greek letters and (lower-case and capital psi (letter), psi, respectively). Wave functions are complex number, complex-valued. For example, a wave function might assign a complex number to each point in a region of space. The Born rule provides the means to turn these complex probability amplitudes into actual probabilities. In one common form, it says that the squared modulus of a wave function that depends upon position is the probability density function, probability density of measurement in quantum mechanics, measuring a particle as being at a given place. The integral of a wavefunction's squared modulus over all the system's degrees of freedom must be equal to 1, a condition called ''normalization''. Since the wave function is complex-valued, only its relative phase and relative magnitud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
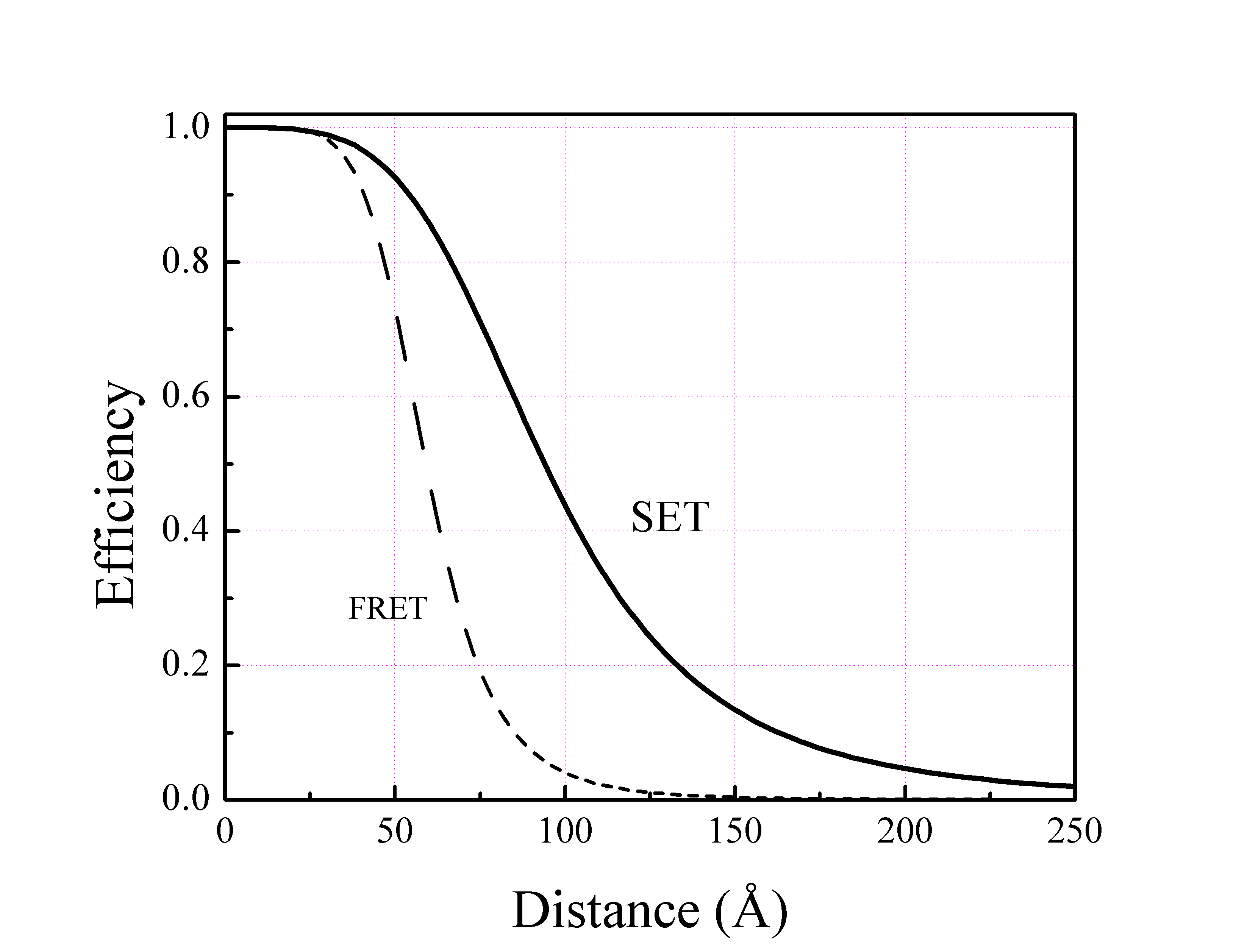
Surface Energy Transfer
Surface energy transfer (SET) is a dipole-surface energy transfer process involving a metallic surface and a molecular dipole. Formula The SET rate follows the inverse of the fourth power of the distance :k_\text = \frac \left(\frac\right)^ where * is the donor emission lifetime; * is the distance between donor-acceptor; * is the distance at which SET efficiency decreases to 50% (i.e., equal probability of energy transfer and spontaneous emission). Efficiency The energy transfer efficiency also follows a similar form :\phi _=\frac Due to the fourth power dependence SET can cover a distance more than 15 nm, which is almost twice the efficiency of FRET. Theoretically predicted in 1978 by Chance ''et al.'' it was proved experimentally in 2000s by different workers. Applications The efficiency of SET as nanoruler has been used in live cells. Gold nano particles are frequently used in these studies as the nanoparticle surface. See also *Dexter electron transfer *Förster resonanc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Förster Resonance Energy Transfer
Förster resonance energy transfer (FRET), fluorescence resonance energy transfer, resonance energy transfer (RET) or electronic energy transfer (EET) is a mechanism describing energy transfer between two light-sensitive molecules (chromophores). A donor chromophore, initially in its electronic excited state, may transfer energy to an acceptor chromophore through nonradiative dipole–dipole coupling. The efficiency of this energy transfer is inversely proportional to the sixth power of the distance between donor and acceptor, making FRET extremely sensitive to small changes in distance. Measurements of FRET efficiency can be used to determine if two fluorophores are within a certain distance of each other. Such measurements are used as a research tool in fields including biology and chemistry. FRET is analogous to Near and far field, near-field communication, in that the radius of interaction is much smaller than the Light, wavelength of light emitted. In the near-field region, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quenching (fluorescence)
In chemistry, quenching refers to any process which decreases the fluorescent intensity of a given substance. A variety of processes can result in quenching, such as excited state reactions, energy transfer, complex-formation and collisions. As a consequence, quenching is often heavily dependent on pressure and temperature. Molecular oxygen, iodine ions and acrylamide are common chemical quenchers. The chloride ion is a well known quencher for quinine fluorescence. Quenching poses a problem for non-instant spectroscopic methods, such as laser-induced fluorescence. Quenching is made use of in optode sensors; for instance the quenching effect of oxygen on certain ruthenium complexes allows the measurement of oxygen saturation in solution. Quenching is the basis for Förster resonance energy transfer (FRET) assays. Quenching and dequenching upon interaction with a specific molecular biological target is the basis for activatable optical contrast agents for molecular imaging. M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission, phosphorescence. Phosphorescent materials continue to emit light for some time after the radiation stops. This difference in duration is a result of quantum spin effects. Fluorescence occurs when a photon from incoming radiation is absorbed by a molecule, exciting it to a higher energy level, followed by the emission of light as the molecule returns to a lower energy state. The emitted light may have a longer wavelength and, therefore, a lower photon energy than the absorbed radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermi's Golden Rule
In quantum physics, Fermi's golden rule is a formula that describes the transition rate (the probability of a transition per unit time) from one energy eigenstate of a quantum system to a group of energy eigenstates in a continuum, as a result of a weak Perturbation theory (quantum mechanics), perturbation. This transition rate is effectively independent of time (so long as the strength of the perturbation is independent of time) and is proportional to the strength of the coupling between the initial and final states of the system (described by the square of the Matrix element (physics), matrix element of the perturbation) as well as the density of states. It is also applicable when the final state is discrete, i.e. it is not part of a continuum, if there is some decoherence in the process, like relaxation or collision of the atoms, or like noise in the perturbation, in which case the density of states is replaced by the reciprocal of the decoherence bandwidth. Historical background ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Van Der Waals Radius
The van der Waals radius, ''r'', of an atom is the radius of an imaginary hard sphere representing the distance of closest approach for another atom. It is named after Johannes Diderik van der Waals, winner of the 1910 Nobel Prize in Physics, as he was the first to recognise that atoms were not simply points and to demonstrate the physical consequences of their size through the van der Waals equation of state. van der Waals volume The van der Waals volume, ''V'', also called the atomic volume or molecular volume, is the atomic property most directly related to the van der Waals radius. It is the volume "occupied" by an individual atom (or molecule). The van der Waals volume may be calculated if the van der Waals radii (and, for molecules, the inter-atomic distances, and angles) are known. For a single atom, it is the volume of a sphere whose radius is the van der Waals radius of the atom:V_ = \pi r_^3. For a molecule, it is the volume enclosed by the van der Waals surf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-radiative Recombination
In solid-state physics of semiconductors, carrier generation and carrier recombination are processes by which mobile charge carriers (electrons and electron holes) are created and eliminated. Carrier generation and recombination processes are fundamental to the operation of many optoelectronic semiconductor devices, such as photodiodes, light-emitting diodes and laser diodes. They are also critical to a full analysis of p-n junction devices such as bipolar junction transistors and p-n junction diodes. The electron–hole pair is the fundamental unit of generation and recombination in inorganic semiconductors, corresponding to an electron transitioning between the valence band and the conduction band where generation of an electron is a transition from the valence band to the conduction band and recombination leads to a reverse transition. Overview Like other solids, semiconductor materials have an electronic band structure determined by the crystal properties of the material. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission, phosphorescence. Phosphorescent materials continue to emit light for some time after the radiation stops. This difference in duration is a result of quantum spin effects. Fluorescence occurs when a photon from incoming radiation is absorbed by a molecule, exciting it to a higher energy level, followed by the emission of light as the molecule returns to a lower energy state. The emitted light may have a longer wavelength and, therefore, a lower photon energy than the absorbed radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound. Electron acceptors are oxidizing agents. The electron accepting power of an electron acceptor is measured by its redox potential. In the simplest case, electron acceptors are reduced by one electron. The process can alter the structure of the acceptor substantially. When the added electron is highly delocalized, the structural consequences of the reduction can be subtle. The central C-C distance in the electron acceptor tetracyanoethylene elongates from 135 to 143 pm upon acceptance of an electron. In the formation of some donor-acceptor complexes, less than one electron is transferred. TTF-TCNQ is a charge transfer complex. Biology In biology, a ''terminal electron acceptor'' often refers to either the last compound to receive an electron in an electron transport chain, such as oxygen during cellular respiration, or the last cofactor to receive an electron within ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Donor
In chemistry, an electron donor is a chemical entity that transfers electrons to another compound. It is a reducing agent that, by virtue of its donating electrons, is itself oxidized in the process. An obsolete definition equated an electron donor and a Lewis base. In contrast to traditional reducing agents, electron transfer from a donor to an electron acceptor may be only fractional. The electron is not completely transferred, which results in an Delocalized electron, electron resonance between the donor and acceptor. This leads to the formation of charge transfer complexes, in which the components largely retain their chemical identities. The electron donating power of a donor molecule is measured by its ionization potential, which is the energy required to remove an electron from the highest occupied molecular orbital (HOMO and LUMO, HOMO). The overall energy balance (ΔE), i.e., energy gained or lost, in an electron donor-acceptor transfer is determined by the difference bet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |