|
Devarda's Alloy
Devarda's alloy (CAS # 8049-11-4) is an alloy of aluminium (44% – 46%), copper (49% – 51%) and zinc (4% – 6%). Devarda's alloy is used as reducing agent in analytical chemistry for the determination of nitrates after their reduction to ammonia under alkaline conditions. It is named for Italian chemist Arturo Devarda (1859–1944), who synthesised it at the end of the 19th century to develop a new method to analyze nitrate in Chile saltpeter. It was often used in the quantitative or qualitative analysis of nitrates in agriculture and soil science before the development of ion chromatography, the predominant analysis method largely adopted worldwide today. General mechanism When a solution of nitrate ions is mixed with aqueous sodium hydroxide, adding Devarda's alloy and heating the mixture gently, liberates ammonia gas. After conversion under the form of ammonia, the total nitrogen is then determined by Kjeldahl method. The reduction of nitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Equation
A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the Product (chemistry), product entities are on the right-hand side with a plus sign between the entities in both the reactants and the products, and an arrow that points towards the products to show the direction of the reaction. The chemical formulas may be symbolic, structural formula, structural (pictorial diagrams), or intermixed. The coefficients next to the symbols and formulas of entities are the absolute values of the Chemical equation#Structure, stoichiometric numbers. The first chemical equation was diagrammed by Jean Beguin in 1615. Structure A chemical equation (see an example below) consists of a list of reactants (the starting substances) on the left-hand side, an arrow (symbol), arrow symbol, and a list of products (substances formed in the chemical reaction) on the right-hand side. Each ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stibine
Stibine (IUPAC name: stibane) is a chemical compound with the formula Sb H3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with H–Sb–H angles of 91.7° and Sb–H distances of 170.7 pm (1.707 Å). The smell of this compound from usual sources (like from reduction of antimony compounds) is reminiscent of arsine, i.e. garlic-like. Preparation SbH3 is generally prepared by the reaction of Sb3+ sources with H− equivalents: :2 Sb2O3 + 3 LiAlH4 → 4 SbH3 + 1.5 Li2O + 1.5 Al2O3 :4 SbCl3 + 3 NaBH4 → 4 SbH3 + 3 NaCl + 3 BCl3 Alternatively, sources of Sb3− react with protonic reagents (even water) to also produce this unstable gas: :Na3Sb + 3 H2O → SbH3 + 3 NaOH Properties The chemical properties of SbH3 resemble those for AsH3. Typical for a heavy hydride (e.g. AsH3, H2Te, SnH4), SbH3 is unstable with respect to its elements. The gas decomposes slowly at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term ''arsine'' is commonly used to describe a class of organoarsenic compounds of the formula AsH3−''x''R''x'', where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine". General properties In its standard state arsine is a colorless, denser-than-air gas that is slightly soluble in water (2% at 20 °C) and in many organic solvents as well. Arsine itself is odorless, but it oxidizes in air and this creates a slight garlic or fish-like scent when the compound is present above 0.5 ppm. This compound is kinetically stable: at room temperature it decomposes only slowly. At temperatures of ca. 230 °C, decomp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nascent Hydrogen
Nascent hydrogen is an outdated concept in organic chemistry that was once invoked to explain dissolving-metal reactions, such as the Clemmensen reduction and the Bouveault–Blanc reduction. Since organic compounds do not react with H2, a special state of hydrogen was postulated. It is now understood that dissolving-metal reactions occur at the metal surface, and the concept of nascent hydrogen has been discredited in organic chemistry. However, the formation of atomic hydrogen is largely invoked in inorganic chemistry and corrosion sciences to explain hydrogen embrittlement in metals exposed to electrolysis and anaerobic corrosion (e.g., dissolution of zinc in strong acids ( HCl) and aluminium in strong bases (NaOH)). The mechanism of hydrogen embrittlement was first proposed by Johnson (1875). The inability of hydrogen atoms to react with organic reagents in organic solvents does not exclude the transient formation of hydrogen atoms capable to immediately diffuse into the cry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite Test
The nitrite ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid. Production Sodium nitrite is made industrially by passing a mixture of nitrogen oxides into aqueous sodium hydroxide or sodium carbonate solution: : : The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid: :2 NH3 + H2O + N2O3 → 2 NH4NO2 Structure The nitrite ion has a symmetrical structure (C2v symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is described as a resonance hybrid with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrate Test
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate. Chemical structure The nitrate anion is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a − charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by three resonance structures: Chemical and biochemical properties In the anion, the oxidation stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gedanken
A thought experiment is an imaginary scenario that is meant to elucidate or test an argument or theory. It is often an experiment that would be hard, impossible, or unethical to actually perform. It can also be an abstract hypothetical that is meant to test our intuitions about morality or other fundamental philosophical questions. History The ancient Greek , "was the most ancient pattern of mathematical proof", and existed before Euclidean mathematics, where the emphasis was on the conceptual, rather than on the experimental part of a thought experiment. Johann Witt-Hansen established that Hans Christian Ørsted was the first to use the equivalent German term . Ørsted was also the first to use the equivalent term in 1820. By 1883, Ernst Mach used in a different sense, to denote exclusively the conduct of a experiment that would be subsequently performed as a by his students. Physical and mental experimentation could then be contrasted: Mach asked his students to provi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reductant
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are common reducing agents include hydrogen, carbon monoxide, the alkali metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/ donates e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term ''arsine'' is commonly used to describe a class of organoarsenic compounds of the formula AsH3−''x''R''x'', where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine". General properties In its standard state arsine is a colorless, denser-than-air gas that is slightly soluble in water (2% at 20 °C) and in many organic solvents as well. Arsine itself is odorless, but it oxidizes in air and this creates a slight garlic or fish-like scent when the compound is present above 0.5 ppm. This compound is kinetically stable: at room temperature it decomposes only slowly. At temperatures of ca. 230 °C, decomp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. Etymology Because it was produced from halite, rock salt according to the methods of Johann Rudolph Glauber, hydrochloric acid was historically called by European alchemists ''spirits of salt'' or ''acidum salis'' (salt acid). Both names are still used, especially in other languages, such as , , , , , , , , , , (''ensan''), zh, 盐酸 (''yánsuān''), and (''yeomsan''). Gaseous HCl was called ''marine acid air''. The name ''muriatic acid'' has the same origin (''muriatic'' means "pertaining to brine or salt", hence ''muriate'' means hydrochloride), and this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
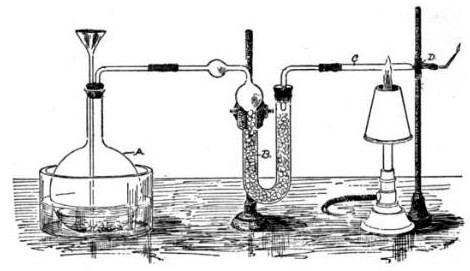
Marsh Test
The Marsh test is a highly sensitive method in the detection of arsenic, especially useful in the field of forensic toxicology when arsenic was used as a poison. It was developed by the chemist James Marsh and first published in 1836. The method continued to be used, with improvements, in forensic toxicology until the 1970s. Arsenic, in the form of white arsenic trioxide , was a highly favored poison, being odourless, easily incorporated into food and drink, and before the advent of the Marsh test, untraceable in the body. In France, it came to be known as ' ("inheritance powder"). For the untrained, arsenic poisoning will have symptoms similar to cholera. Precursor methods The first breakthrough in the detection of arsenic poisoning was in 1775 when Carl Wilhelm Scheele discovered a way to change arsenic trioxide to garlic-smelling arsine gas (), by treating it with nitric acid () and combining it with zinc: : In 1787, German physician (1739-1805) discovered that if arseni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |