|
Depsipeptides
A depsipeptide is a peptide in which one or more of its amide, -C(O)NHR-, groups are replaced by the corresponding ester, -C(O)OR-. Many depsipeptides have both peptide and ester linkages. Elimination of the N–H group in a peptide structure results in a decrease of H-bonding capability, which is responsible for secondary structure and folding patterns of peptides, thus inducing structural deformation of the helix and β-sheet structures. Because of decreased resonance delocalization in esters relative to amides, depsipeptides have lower rotational barriers for cis-trans isomerization and therefore they have more flexible structures than their native analogs. They are mainly found in marine and microbial natural products. Depsipeptide natural products 222px, Enterochelin is a depsipeptide that is an iron-transporter. Several depsipeptides have been found to exhibit anti-cancer properties. A depsipeptide enzyme inhibitor includes romidepsin, a member of the bicyclic peptide clas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mirabamide
Mirabamides are sea sponge Sponges or sea sponges are primarily marine invertebrates of the animal phylum Porifera (; meaning 'pore bearer'), a basal clade and a sister taxon of the diploblasts. They are sessile filter feeders that are bound to the seabed, and a ... isolates that inhibit HIV-1 fusion. Variants A to D are known from ''Siliquariaspongia mirabilis'' and E through H are derived from ''Stelletta clavosa''. Mirabamides have a macrocyclic region closed through an ester bond between the C-terminus and a 𝛽-hydroxyl group, and terminated with a polyketide moiety or a more simple branched aliphatic acid. Mirabamide G is a fusion of several amino acids: 2,3 diamino butanoic acid, 3-hydroxyleucine, N-methylthreonine, 2,3-dihydroxy-2,6,8-trimethyldeca-(4''Z'',6''E'')-dienoic acid, 3-methoxy alanine, β-methoxytyrosine, 3,4-dimethylglutamine. References Depsipeptides {{organic-compound-stub ''attribution'': Contains text from the CC/BY/4.0 licensed " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Papuamide
Papuamides A and B are depsipeptides which appear to protect T cells from HIV. They were isolated from the sponge Theonella, and are part of a larger group of structurally similar depsipeptides—also isolated from sponges—including neamphamide A Neamphamide A is an HIV-inhibitory isolate of the sea sponge '' Neamphius huxleyi''. References Depsipeptides {{organic-compound-stub ..., callipeltin A, and mirabamides A-D. References Depsipeptides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Callipeltin
Callipeltin A and B are depsipeptides isolated from marine invertebrate Invertebrates are animals that neither develop nor retain a vertebral column (commonly known as a ''spine'' or ''backbone''), which evolved from the notochord. It is a paraphyletic grouping including all animals excluding the chordata, chordate s ...s. Preliminary research shows that they may have antiviral activity. See also * Papuamide References Depsipeptides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Romidepsin
Romidepsin, sold under the brand name Istodax, is an anticancer agent used in cutaneous T-cell lymphoma (CTCL) and other peripheral T-cell lymphomas (PTCLs). Romidepsin is a natural product obtained from the bacterium ''Chromobacterium violaceum'', and works by blocking enzymes known as histone deacetylases, thus inducing apoptosis. It is sometimes referred to as depsipeptide, after the class of molecules to which it belongs. Romidepsin is branded and owned by Gloucester Pharmaceuticals, a part of Celgene. History Romidepsin was first reported in the scientific literature in 1994, by a team of researchers from Fujisawa Pharmaceutical Company (now Astellas Pharma) in Tsukuba, Japan, who isolated it in a culture of ''Chromobacterium violaceum'' from a soil sample obtained in Yamagata Prefecture. It was found to have little to no antibacterial activity, but was potently cytotoxic against several human cancer cell lines, with no effect on normal cells; studies on mice later found it ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptogramin B
Streptogramin B is a subgroup of the streptogramin antibiotics family. These natural products are cyclic hexa- or hepta depsipeptides produced by various members of the genus of bacteria ''Streptomyces''. Many of the members of the streptogramins reported in the literature have the same structure and different names; for example, pristinamycin IA = vernamycin Bα = mikamycin B = osteogrycin B. Biosynthesis The biosynthesis of streptogramin B is carried out by large multifunctional enzymes called non-ribosomal peptide synthetases (NRPS). In the NRPS system, each amino acid is activated as an aminoacyladenylate and is linked to the enzyme as a thioester with a phosphopantetheinyl group. An elongation reaction then occurs by transferring the activated carboxyl to the amino group in the next amino acid, thus executing the N-to-C stepwise condensation. NRPSs contain several modules on a single polypeptide. Each of these modules can catalyze activation, condensation and a modification rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides are short chains of amino acids linked by peptide bonds. A polypeptide is a longer, continuous, unbranched peptide chain. Polypeptides that have a molecular mass of 10,000 Da or more are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. Peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyclic peptides have an N-terminal (amine group) and C-terminal (carboxyl g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyldepsipeptide Antibiotics
Acyldepsipeptide or cyclic acyldepsipeptide (ADEP) is a class of potential antibiotics first isolated from bacteria and act by deregulating the ClpP protease. Natural ADEPs were originally found as products of aerobic fermentation in '' Streptomyces hawaiiensis'', A54556A and B, and in the culture broth of ''Streptomyces'' species, enopeptin A and B. ADEPs are of great interest in drug development due to their antibiotic properties and thus are being modified in attempt to achieve greater antimicrobial activity. The potential role of ADEPs in combating antibiotic drug resistance is postulated due to their novel mode of action that other antibiotics are not known to use, activation of casein lytic protease ( ClpP) which is an important bacterial protease. Most antibiotics work through inhibitory processes to establish cell death, while ADEPs actually work through activation of the protease to cause uncontrolled protein degradation, inhibition of cell division, and subsequent cell de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Passerini Reaction
The Passerini reaction is a chemical reaction involving an isocyanide, an aldehyde (or ketone), and a carboxylic acid to form a α-acyloxy amide. This addition reaction is one of the oldest isocyanide-based multicomponent reactions and was first described in 1921 by Mario Passerini in Florence, Italy. It is typically carried out in Polar aprotic solvent, aprotic solvents but can alternatively be performed in water, ionic liquids, or deep eutectic solvents. It is a third order reaction; first order in each of the reactants. The Passerini reaction is often used in Combinatorial chemistry, combinatorial and medicinal chemistry with recent utility in green chemistry and polymer chemistry. As isocyanides exhibit high functional group tolerance, chemoselectivity, regioselectivity, and stereoselectivity, the Passerini reaction has a wide range of synthetic applications.''The Passirini Reaction'' L. Banfi, R.Riva in Organic Reactions vol. 65 L.E. Overman Ed. Wiley 2005 Mechanism The Pass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
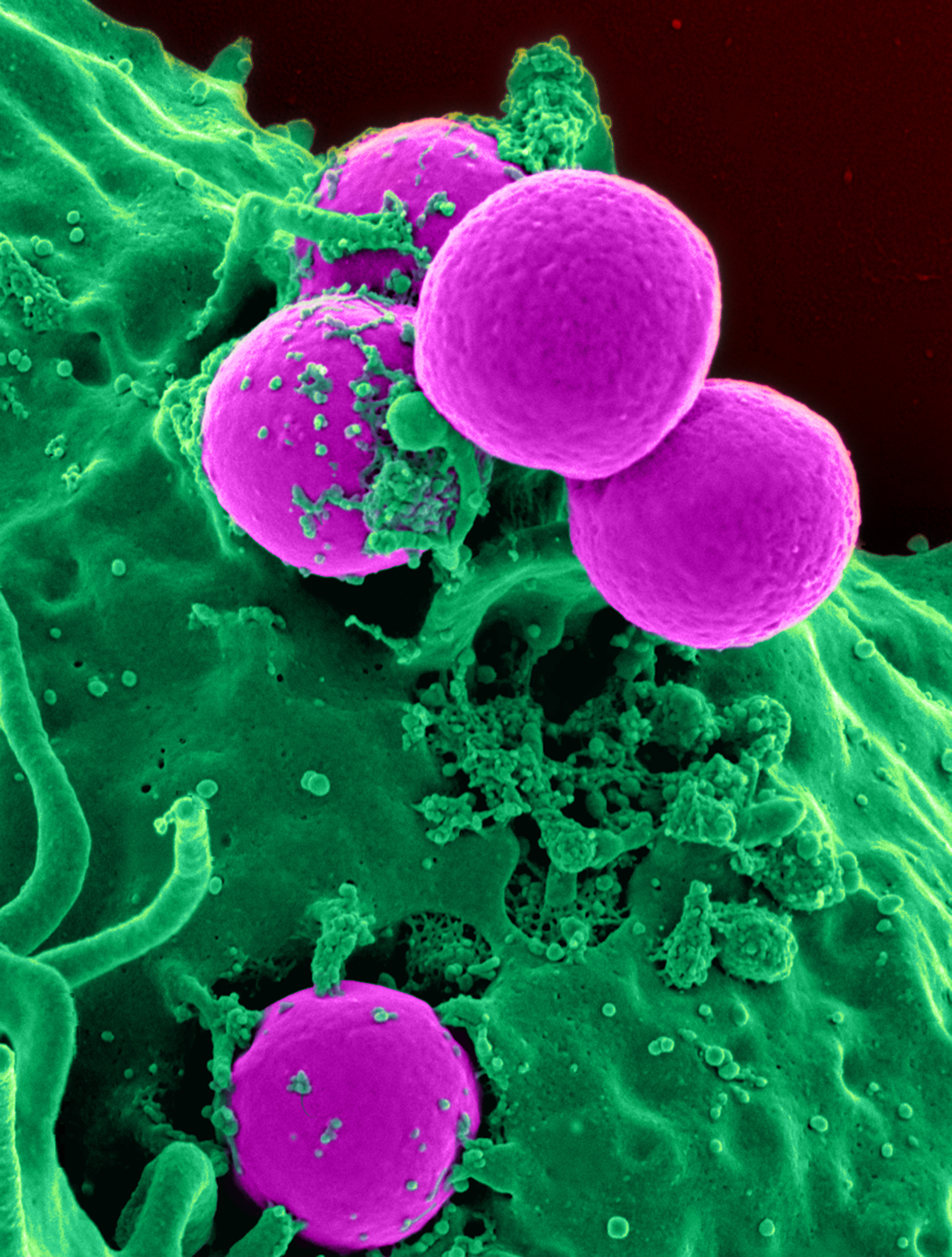
Methicillin-resistant Staphylococcus Aureus
Methicillin-resistant ''Staphylococcus aureus'' (MRSA) is a group of gram-positive bacteria that are genetically distinct from other strains of ''Staphylococcus aureus''. MRSA is responsible for several difficult-to-treat infections in humans. It caused more than 100,000 deaths worldwide attributable to antimicrobial resistance in 2019. MRSA is any strain of ''S. aureus'' that has developed (through mutation) or acquired (through horizontal gene transfer) a multiple drug resistance to beta-lactam antibiotics. Beta-lactam (β-lactam) antibiotics are a broad-spectrum group that include some penams (penicillin derivatives such as methicillin and oxacillin) and cephems such as the cephalosporins. Strains unable to resist these antibiotics are classified as methicillin-susceptible ''S. aureus'', or MSSA. MRSA infection is common in hospitals, prisons, and nursing homes, where people with open wounds, invasive devices such as catheters, and weakened immune systems are at greate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |