|
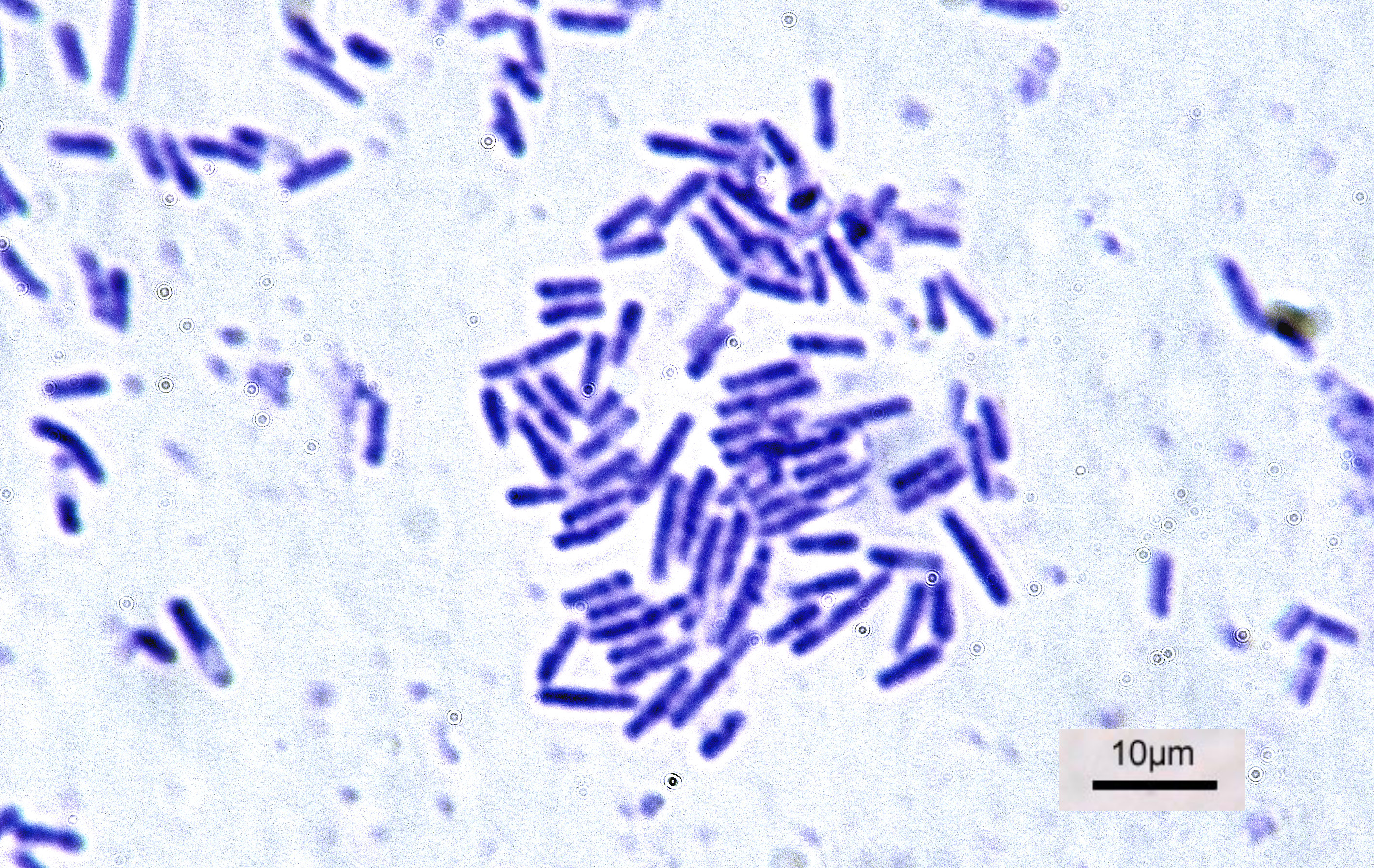
Dehalococcoides Mccartyi
''Dehalococcoides'' is a genus of bacteria within class Dehalococcoidia that obtain energy via the oxidation of hydrogen and subsequent reductive dehalogenation of halogenated organic compounds in a mode of anaerobic respiration called organohalide respiration. They are well known for their great potential to remediate halogenated ethenes and aromatics. They are the only bacteria known to transform highly chlorinated dioxins, PCBs. In addition, they are the only known bacteria to transform tetrachloroethene ( perchloroethene, PCE) to ethene. Microbiology The first member of the genus ''Dehalococcoides'' was described in 1997 as ''Dehalococcoides ethenogenes'' strain 195 ( nom. inval.). Additional ''Dehalococcoides'' members were later described as strains CBDB1, BAV1, FL2, VS, and GT. In 2012 all yet-isolated ''Dehalococcoides'' strains were summarized under the new taxonomic name '' D. mccartyi'', with strain 195 as the type strain. GTDB release 202 clusters the genus into th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteria
Bacteria (; singular: bacterium) are ubiquitous, mostly free-living organisms often consisting of one biological cell. They constitute a large domain of prokaryotic microorganisms. Typically a few micrometres in length, bacteria were among the first life forms to appear on Earth, and are present in most of its habitats. Bacteria inhabit soil, water, acidic hot springs, radioactive waste, and the deep biosphere of Earth's crust. Bacteria are vital in many stages of the nutrient cycle by recycling nutrients such as the fixation of nitrogen from the atmosphere. The nutrient cycle includes the decomposition of dead bodies; bacteria are responsible for the putrefaction stage in this process. In the biological communities surrounding hydrothermal vents and cold seeps, extremophile bacteria provide the nutrients needed to sustain life by converting dissolved compounds, such as hydrogen sulphide and methane, to energy. Bacteria also live in symbiotic and parasitic re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Viologen
Paraquat (trivial name; ), or ''N'',''N''′-dimethyl-4,4′-bipyridinium dichloride (systematic name), also known as methyl viologen, is an organic compound with the chemical formula C6H7N)2l2. It is classified as a viologen, a family of redox-active heterocycles of similar structure. This salt is one of the most widely used herbicides. It is quick-acting and non-selective, killing green plant tissue on contact. It is also toxic (lethal) to human beings and animals due to its redox activity, which produces superoxide anions. It has been linked to the development of Parkinson's disease and is banned in several countries. Paraquat may be in the form of salt with chloride or other anions; quantities of the substance are sometimes expressed by cation mass alone (paraquat cation, paraquat ion). The name is derived from the ''para'' positions of the ''quaternary'' nitrogens. Production Pyridine is coupled by treatment with sodium in ammonia followed by oxidation to give 4,4′ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polychlorinated Biphenyl
Polychlorinated biphenyls (PCBs) are highly carcinogenic chemical compounds, formerly used in industrial and consumer products, whose production was banned in the United States by the Toxic Substances Control Act in 1979 and internationally by the Stockholm Convention on Persistent Organic Pollutants in 2001. They are organic chlorine compounds with the formula C12 H10−''x'' Cl''x''; they were once widely used in the manufacture of carbonless copy paper, as heat transfer fluids, and as dielectric and coolant fluids for electrical equipment. Because of their longevity, PCBs are still widely in use, even though their manufacture has declined drastically since the 1960s, when a host of problems were identified. With the discovery of PCBs' environmental toxicity, and classification as persistent organic pollutants, their production was banned by United States federal law in 1978, and by the Stockholm Convention on Persistent Organic Pollutants in 2001. The International Ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzenes
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structure, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. History Discovery The word "''benzene''" derives from "''gum benzoin''" (benzoin resi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polychlorinated Dibenzodioxins
Polychlorinated dibenzodioxins (PCDDs), or simply dioxins, are a group of long-lived polyhalogenated organic compounds that are primarily anthropogenic, and contribute toxic, persistent organic pollution in the environment. They are commonly but inaccurately referred to as dioxins for simplicity, because every PCDD molecule contains a dibenzo-1,4-dioxin skeletal structure, with 1,4-dioxin as the central ring. Members of the PCDD family bioaccumulate in humans and wildlife because of their lipophilic properties, and may cause developmental disturbances and cancer. Because dioxins can persist in the environment for more than 100 years, the majority of PCDD pollution today is not the result of recent emissions, but the cumulative result of synthetic processes undertaken since the beginning of the 20th century, including organochloride-related manufacturing, incineration of chlorine-containing substances such as polyvinyl chloride (PVC), and chlorine bleaching of paper. Fore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule. Phenols are both synthesized industrially and produced by plants and microorganisms. Properties Acidity Phenols are more acidic than typical alcohols. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12). Deprotonation of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides (aryloxides according to the IUPAC Gold Book). Condensation with aldehydes and ketones Phenols are susceptible to Electrophilic aromatic substitutions. Condensation with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polychlorinated Biphenyl
Polychlorinated biphenyls (PCBs) are highly carcinogenic chemical compounds, formerly used in industrial and consumer products, whose production was banned in the United States by the Toxic Substances Control Act in 1979 and internationally by the Stockholm Convention on Persistent Organic Pollutants in 2001. They are organic chlorine compounds with the formula C12 H10−''x'' Cl''x''; they were once widely used in the manufacture of carbonless copy paper, as heat transfer fluids, and as dielectric and coolant fluids for electrical equipment. Because of their longevity, PCBs are still widely in use, even though their manufacture has declined drastically since the 1960s, when a host of problems were identified. With the discovery of PCBs' environmental toxicity, and classification as persistent organic pollutants, their production was banned by United States federal law in 1978, and by the Stockholm Convention on Persistent Organic Pollutants in 2001. The International Ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Chloride
Vinyl chloride is an organochloride with the formula H2C=CHCl. It is also called vinyl chloride monomer (VCM) or chloroethene. This colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride (PVC). About 13 million metric tonne are produced annually. VCM is among the top twenty largest petrochemicals (petroleum-derived chemicals) in world production. The United States currently remains the largest VCM manufacturing region because of its low-production-cost position in chlorine and ethylene raw materials. China is also a large manufacturer and one of the largest consumers of VCM. Vinyl chloride is a gas with a sweet odor. It is highly toxic, flammable, and carcinogenic. It can be formed in the environment when soil organisms break down chlorinated solvents. Vinyl chloride that is released by industries or formed by the breakdown of other chlorinated chemicals can enter the air and drinking water supplies. Vinyl chloride is a common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trichloroethene
The chemical compound trichloroethylene is a halocarbon commonly used as an industrial solvent. It is a clear, colourless non-flammable liquid with a chloroform-like sweet smell. It should not be confused with the similar 1,1,1-trichloroethane, which is commonly known as ''chlorothene''. The IUPAC name is trichloroethene. Industrial abbreviations include TCE, trichlor, Trike, Tricky and tri. It has been sold under a variety of trade names. Under the trade names Trimar and Trilene, trichloroethylene was used as a volatile anesthetic and as an inhaled obstetrical analgesic in millions of patients. Groundwater and drinking water contamination from industrial discharge including trichloroethylene is a major concern for human health and has precipitated numerous incidents and lawsuits. History Pioneered by Imperial Chemical Industries in Britain, its development was hailed as an anesthetic revolution. Originally thought to possess less hepatotoxicity than chloroform, and without the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachloroethene
Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and many other names (and abbreviations such as "perc" or "PERC", and "PCE"), is a chlorocarbon with the formula Cl2C=CCl2 . It is a colorless liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid". It also has its uses as an effective automotive brake cleaner. It has a sweet odor detectable by most people at a concentration of 1 part per million (1 ppm). Worldwide production was about in 1985.M. Rossberg et al. "Chlorinated Hydrocarbons" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2006, Wiley-VCH, Weinheim. Production British physicist and chemist Michael Faraday first synthesized tetrachloroethylene in 1821 by thermal decomposition of hexachloroethane. :C2Cl6 → C2Cl4 + Cl2 Most tetrachloroethylene is produced by high temperature chlorinolysis of light hydrocarbons. The method is related to Faraday's discovery since hexachl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetate
An acetate is a salt formed by the combination of acetic acid with a base (e.g. alkaline, earthy, metallic, nonmetallic or radical base). "Acetate" also describes the conjugate base or ion (specifically, the negatively charged ion called an anion) typically found in aqueous solution and written with the chemical formula . The neutral molecules formed by the combination of the acetate ion and a ''positive'' ion (called a cation) are also commonly called "acetates" (hence, ''acetate of lead'', ''acetate of aluminum'', etc.). The simplest of these is hydrogen acetate (called acetic acid) with corresponding salts, esters, and the polyatomic anion , or . Most of the approximately 5 billion kilograms of acetic acid produced annually in industry are used in the production of acetates, which usually take the form of polymers. In nature, acetate is the most common building block for biosynthesis. Nomenclature and common formula When part of a salt, the formula of the acetate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)
2.png)

