|
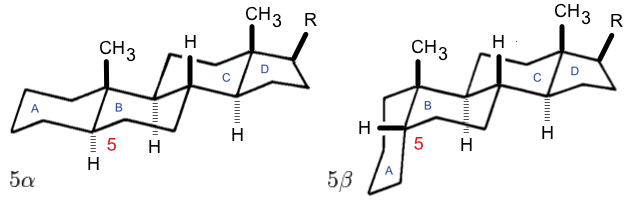
Dammarane
Dammarane is a tetracyclic triterpene found in sapogenins (forming triterpenoid saponins) like those of ginseng (ginsenosides: panaxatriol Panaxatriol is an organic compound that is an aglycone of ginsenosides, a group of steroid glycosides. It is a dammarane-type tetracyclic triterpene sapogenin found in ginseng (''Panax ginseng'') and in notoginseng (''Panax pseudoginseng''). It ... and protopanaxadiol). Compounds of the series were first isolated from and named after dammar resin, a natural resin from the tropical trees of the dipterocarp family. Mills J.S. (1956) "The Constitution of the Neutral, Tetracyclic Triterpenes of Dammar Resin" ''Journal of the Chemical Society'' 2196-2202 References External links Numbering of dammarane according to IUPAC Recommendations Steroids Triterpenes {{steroid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpene
Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' often are used i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sapogenin
Sapogenins are aglycones (non-saccharide moieties) of saponins, a large family of natural products. Sapogenins contain steroid or other triterpene frameworks as their key organic feature. For example, steroidal sapogenins such as tiggenin, neogitogenin, and tokorogenin have been isolated from the tubers of '' Chlorophytum arundinaceum''. Some steroidal sapogenins can serve as a practical starting point for the semisynthesis Semisynthesis, or partial chemical synthesis, is a type of chemical synthesis that uses chemical compounds isolated from natural sources (such as microbiology, microbial cell cultures or plant material) as the starting materials to produce novel ... of particular steroid hormones. Diosgenin and hecogenin are other examples of sapogenins. References Phytochemicals Triterpenes Alcohols {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpenoid
Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' often are used i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saponin
Saponins (Latin ''sapon'', 'soap' + ''-in'', 'one of') are bitter-tasting, usually toxic plant-derived secondary metabolites. They are organic chemicals that become foamy when agitated in water and have high molecular weight. They are present in a wide range of plant species throughout the bark, leaves, stems, roots and flowers but particularly in soapwort (genus '' Saponaria''), a flowering plant, the soapbark tree ('' Quillaja saponaria''), common corn-cockle ('' Agrostemma githago'' L.), baby's breath ( ''Gypsophila'' spp.) and soybeans ('' Glycine max'' L.). They are used in soaps, medicines (e.g. drug adjuvants), fire extinguishers, dietary supplements, steroid synthesis, and in carbonated beverages (for example, being responsible for maintaining the head on root beer). Saponins are both water and fat soluble, which gives them their useful soap properties. Some examples of these chemicals are glycyrrhizin ( licorice flavoring) and quillaia (alt. quillaja), a bark ext ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ginsenoside
Ginsenosides or panaxosides are a class of natural product steroid glycosides and triterpene saponins. Compounds in this family are found almost exclusively in the plant genus ''Panax'' (ginseng), which has a long history of use in traditional medicine that has led to the study of pharmacological effects of ginseng compounds. As a class, ginsenosides exhibit a large variety of subtle and difficult-to-characterize biological effects when studied in isolation. Ginsenosides can be isolated from various parts of the plant, though typically from the roots, and can be purified by column chromatography. The chemical profiles of ''Panax'' species are distinct; although Asian ginseng, ''Panax ginseng'', has been most widely studied due to its use in traditional Chinese medicine, there are ginsenosides unique to American ginseng (''Panax quinquefolius'') and Japanese ginseng (''Panax japonicus''). Ginsenoside content also varies significantly due to environmental effects. The leaves and stem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Panaxatriol
Panaxatriol is an organic compound that is an aglycone of ginsenosides, a group of steroid glycosides. It is a dammarane-type tetracyclic triterpene sapogenin found in ginseng (''Panax ginseng'') and in notoginseng (''Panax pseudoginseng''). It is formed by the dehydration reaction, dehydration of protopanaxatriol. See also * Protopanaxadiol References {{alcohol-stub Triterpenes Tetrahydropyrans Triols Sterols ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protopanaxadiol
Protopanaxadiol (PPD) is an organic compound that is an aglycone of ginsenosides, a group of steroid glycosides. It is a dammarane-type tetracyclic terpene sapogenin found in ginseng (''Panax ginseng'') and in notoginseng (''Panax pseudoginseng''). The health effect of protopanaxadiol inside the human body is still unclear. One study suggests it has rapid, non-genomic effects on endothelial cells, binding to the glucocorticoid and oestrogen beta receptors. The study also showed an increase in intracellular calcium ion concentration. Shanghai Innovative Research Center of Traditional Chinese Medicine (SIRC-TCM) is trying to develop an antidepressant based on this substance under the brand name ''Yoxintine''. The drug has finished phase II trials as of May 2023. Results were published in November 2024. A pharmacokinetic study done on phase IIa human volunteers reports the relationship between oral dose and blood plasma concentration, but no data is given for oral bioavailbilit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dammar Resin
Dammar, also called dammar gum, or damar gum, is a resin obtained from the tree family Dipterocarpaceae in India and Southeast Asia, principally those of the genera ''Shorea'' or '' Hopea'' (synonym ''Balanocarpus''). The resin of some species of '' Canarium'' may also called dammar. Most is produced by tapping trees; however, some is collected in fossilised form on the ground. The gum varies in colour from clear to pale yellow, while the fossilised form is grey-brown. Dammar gum is a triterpenoid resin, containing many triterpenes and their oxidation products. Many of them are low molecular weight compounds (dammarane, dammarenolic acid, oleanane, oleanonic acid, etc.), which easily oxidizes and photoxidizes. Types * ''Damar mata kucing'' ('cat's eye damar') is a crystalline resin, usually in the form of round balls. '' Shorea javanica'' is an important source in Indonesia. * ''Damar batu'' ('stone damar') is stone or pebble-shaped, opaque dammar collected from the ground ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dipterocarp
Dipterocarpaceae is a family of flowering plants with 22 genera and about 695 known species of mainly lowland tropical forest trees. Their distribution is pantropical, from northern South America to Africa, the Seychelles, India, Indochina, Indonesia, Malaysia and Philippines. The greatest diversity of Dipterocarpaceae occurs in Borneo.Ashton, P.S. Dipterocarpaceae. ''Flora Malesiana'', 1982 Series I, 92: 237-552 The largest genera are ''Shorea'' (196 species), '' Hopea'' (104 species), ''Dipterocarpus'' (70 species), and ''Vatica'' (65 species).Ashton, P.S. Dipterocarpaceae. In ''Tree Flora of Sabah and Sarawak,'' Volume 5, 2004. Soepadmo, E., Saw, L. G. and Chung, R. C. K. eds. Government of Malaysia, Kuala Lumpur, Malaysia. Many are large forest-emergent species, typically reaching heights of 40–70 m, some even over 80 m (in the genera '' Dryobalanops'', '' Hopea'' and ''Shorea''), with the tallest known living specimen (''Shorea faguetiana'') 93.0 m tall. Named Menara, 't ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroids
A steroid is an organic compound with four fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in fungi, plants, and animals. All steroids are manufactured in cells from a sterol: cholesterol (animals), lanosterol ( opisthokonts), or cycloartenol (plants). All three of these molecules are produced via cyclization of the triterpene squalene. Structure The steroid nucleus ( core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in four fused rings: three six-member cyclohexane rings (rings A, B and C in the first illus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |