|
Cyclobutene
Cyclobutene is a cycloalkene. It is of interest in research but currently has no practical applications. It is a colorless easily condensed gas. A modern synthesis involves the 2-step dehydration of cyclobutanol. The compound was first prepared by thermolysis of the ammonium salt 4H7NMe3H. Cyclobutene thermally isomerizes to 1,3-butadiene. This strongly exothermic reaction reflects the dominance of ring strain. In contrast, the corresponding equilibrium for hexafluorocyclobutene disfavors hexafluorobutadiene. See also * Cyclobutane * Cyclobutadiene * Cyclobutyne * Squaric acid Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2. The conjugate base of squaric acid is the hydrogensquarate anion ; and the conjug ... References Monomers {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Squaric Acid
Squaric acid, also called quadratic acid because its four carbon atoms approximately form a square, is a diprotic organic acid with the chemical formula C4O2(OH)2. The conjugate base of squaric acid is the hydrogensquarate anion ; and the conjugate base of the hydrogensquarate anion is the divalent squarate anion . This is one of the oxocarbon anions, which consist only of carbon and oxygen. Squaric acid is a reagent for chemical synthesis, used for instance to make photosensitive squaraine dyes and inhibitors of protein tyrosine phosphatases. Chemical properties Squaric acid is a white crystalline powder. The onset of thermal decomposition depends on the different thermodynamic conditions such as heating rates. The structure of squaric acid is not a perfect square, as the carbon–carbon bond lengths are not quite equal. The high acidity with p''K''a = 1.5 for the first proton and p''K''a = 3.4 for the second is attributable to resonance stabilization of the anion. Because t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
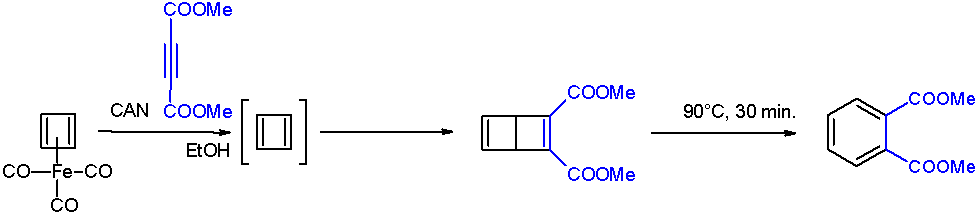
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 π-electrons. It is the smallest 'n''annulene ( annulene). Its rectangular structure is the result of the Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different 1,2-dideutero-1,3-cyclobutadiene valence iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloalkene
A cycloalkene or cycloolefin is a type of alkene hydrocarbon which contains a closed ring of carbon Atom, atoms and either one or more double bonds, but has no Aromaticity, aromatic character. Some cycloalkenes, such as cyclobutene and cyclopentene, can be used as Monomer, monomers to produce polymer chains. Due to geometrical considerations, smaller cycloalkenes are almost always the Cis–trans isomerism, ''cis'' isomers, and the term ''cis'' tends to be omitted from the names. Cycloalkenes require considerable p-orbital overlap in the form of a bridge between the carbon-carbon double bond, however, this is not feasible in smaller molecules due to the increase of strain that could break the molecule apart. In greater carbon number cycloalkenes, the addition of CH2 substituents decreases strain. trans-Cycloalkenes with 7 or fewer carbons in the ring will not occur under normal conditions because of the large amount of ring strain needed. In larger rings (8 or more atoms), Cis–tran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial or biological significance, but more complex derivatives are important in biology and biotechnology. Structure The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. butane or cyclohexane. As such, cyclobutane is unstable above about 500 °C. The four carbon atoms in cyclobutane are not coplanar; instead the ring typically adopts a folded or "puckered" conformation. This implies that the C-C-C angle is less than 90°. One of the carbon atoms makes a 25° angle with the plane formed by the other three carbons. In this way some of the eclipsing interactions are reduced. The conformation is also known as a "butterfly". Equivalent p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutyne
Cyclobutyne (C4H4) is a hydrocarbon molecule containing a triple bond within a four carbon atom ring. This cycloalkyne is very unstable due to its high ring strain and has not been isolated in the pure state. However, osmium coordination complexes containing cyclobutyne have been synthesized. See also *Cyclobutene Cyclobutene is a cycloalkene. It is of interest in research but currently has no practical applications. It is a colorless easily condensed gas. A modern synthesis involves the 2-step dehydration of cyclobutanol. The compound was first prepare ... References Alkynes Four-membered rings {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutanol
Cyclobutanol is an organic compound with the chemical formula C4H8O. It contains a cyclobutyl group with a hydroxyl In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ... group attached to it. References {{reflist Cycloalkanols Cyclobutyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction. Decomposition temperature definition A simple substance (like water) may exist in equilibrium with its thermal decomposition products, effectively halting the decomposition. The equilibrium fraction of decomposed molecules increases with the temperature. Examples * Calcium carbonate (limestone or chalk) decomposes into calcium oxide and carbon dioxide when heated. The chemical reaction is as follows: ::CaCO3 → CaO + CO2 :The reaction is used to make Calcium oxide, quick lime, which is an industrially impor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorocyclobutene
Hexafluorocyclobutene is the organofluorine compound with the formula (CF2)2(CF)2. A colorless gas, it is a precursor to a variety of compounds, including squaric acid. Hexafluorocyclobutene is prepared in two steps from chlorotrifluoroethylene. The thermal dimerization gives 1,2-dichloro-1,2,3,3,4,4-hexafluorocyclobutane. Dechlorination of the latter gives hexafluorocyclobutene: :C4F6Cl2 + Zn -> C4F6 + ZnCl2 Safety Reminiscent of perfluoroisobutene, hexafluorocyclobutene is quite toxic with an LD = 6000 mg/min/m−3 (mice).{{cite book , doi=10.1016/B978-008043405-6/50040-2, chapter=Highly-toxic fluorine compounds, title=Fluorine Chemistry at the Millennium, year=2000, last1=Timperley, first1=Christopher M., pages=499–538, isbn=9780080434056 See also *Hexafluoro-2-butyne *Hexafluorobutadiene Hexafluorobutadiene is an organofluorine compound with the formula (CF2=CF)2. A colorless gas, it has attracted attention as an etchant in microelectronics. It is the perfluor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorobutadiene
Hexafluorobutadiene is an organofluorine compound with the formula (CF2=CF)2. A colorless gas, it has attracted attention as an etchant in microelectronics. It is the perfluoroanalogue of butadiene. It can be prepared by coupling of C2 compounds such as from chlorotrifluoroethylene or bromotrifluoroethylene. Routes from C4 species have also been demonstrated. For example, an early synthesis involved Zn-induced dechlorinaion of 1,2,3,4-tetrachloro-1,1,2,3,4,4-hexafluorobutane. Hexafluorobutadiene dimerizes via a +2process at 150 °C to give perfluorinated divinylcyclobutanes.{{cite book , doi=10.1002/0470864028.ch21 , title=The Chemistry of Cyclobutanes , chapter=Fluorinated Cyclobutanes and Their Derivatives, first1=David M., last1=Lemal, first2=Xudong, last2=Chen , editor=Zvi Rappoport , editor2=Joel F. Liebman, year=2005, series=PATAI'S Chemistry of Functional Groups, pages=955–1029 , isbn=0470864001 See also * Hexafluoro-2-butyne, an isomer of C4F6 * Hexafluorocyclo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomers
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |