|
Cartridge Heater
A cartridge heater is a tube-shaped, heavy-duty, industrial Joule heating element used in the process heating industry, usually custom manufactured to a specific watt density, based on its intended application. Compact designs are capable of reaching a watt density of up to 50W/cm² while some specialty high temperature designs can reach 100w/cm². Applications Cartridge heaters are found useful in many applications, such as: * Seal bars *Torpedo heaters for injection molding * Injection molding manifolds * Mass spectrometry * Rubber molding * Food production * Immersion tank heating * HVAC compressors * Fuel cells * Semiconductors * Medical devices * Sensor measurement devices * Extrusion * Die casting * Hot melt adhesives * Heat staking / hole punch * Plastic welding * Fluid heating * 3D Printers File:Cartridge-heater-cold.jpg, Cold cartridge heater File:Cartridge-heater-hot.jpg, Hot cartridge heater Construction Construction of a cartridge heater may be divided in 7 m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joule Heating
Joule heating, also known as resistive, resistance, or Ohmic heating, is the process by which the passage of an electric current through a conductor produces heat. Joule's first law (also just Joule's law), also known in countries of former USSR as the Joule–Lenz law, Assuming the element behaves as a perfect resistor and that the power is completely converted into heat, the formula can be re-written by substituting Ohm's law, V = I R , into the generalized power equation: P = IV = I^2R = V^2/R where ''R'' is the resistance. Alternating current When current varies, as it does in AC circuits, P(t) = U(t) I(t) where ''t'' is time and ''P'' is the instantaneous power being converted from electrical energy to heat. Far more often, the ''average'' power is of more interest than the instantaneous power: P_ = U_\text I_\text = I_\text^2 R = U_\text^2 / R where "avg" denotes average (mean) over one or more cycles, and "rms" denotes root mean square. These formulas are valid fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nichrome
Nichrome (also known as NiCr, nickel-chromium or chromium-nickel) is a family of alloys of nickel, chromium, and often iron (and possibly other elements) commonly used as resistance wire, heating elements in devices like toasters, electrical kettles and space heaters, in some dental restorations (fillings) and in a few other applications. Patented in 1906 by Albert Marsh (US patent 811,859), nichrome is the oldest documented form of resistance heating alloy. A common nichrome alloy is 80% nickel and 20% chromium by mass, but there are many other combinations of metals for various applications. Properties Nichrome is consistently silvery-grey in colour, is corrosion-resistant, has a high melting point of about , and has an electrical resistivity of around 112 microOhm-cm, which is around 66 times higher resistivity than copper of 1.678 microOhm-cm. Almost any conductive wire can be used for heating, but most metals conduct electricity with great efficiency, requiring them ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from ''magnesia negra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a model s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stainless Steel
Stainless steel is an alloy of iron that is resistant to rusting and corrosion. It contains at least 11% chromium and may contain elements such as carbon, other nonmetals and metals to obtain other desired properties. Stainless steel's resistance to corrosion results from the chromium, which forms a passive film that can protect the material and self-heal in the presence of oxygen. The alloy's properties, such as luster and resistance to corrosion, are useful in many applications. Stainless steel can be rolled into sheets, plates, bars, wire, and tubing. These can be used in cookware, cutlery, surgical instruments, major appliances, vehicles, construction material in large buildings, industrial equipment (e.g., in paper mills, chemical plants, water treatment), and storage tanks and tankers for chemicals and food products. The biological cleanability of stainless steel is superior to both aluminium and copper, having a biological cleanability comparable to glass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Superalloy
A superalloy, or high-performance alloy, is an alloy with the ability to operate at a high fraction of its melting point. Several key characteristics of a superalloy are excellent mechanical strength, resistance to thermal creep deformation, good surface stability, and resistance to corrosion or oxidation. The crystal structure is typically face-centered cubic (FCC) austenitic. Examples of such alloys are Hastelloy, Inconel, Waspaloy, Rene alloys, Incoloy, MP98T, TMS alloys, and CMSX single crystal alloys. Superalloy development has relied heavily on both chemical and process innovations. Superalloys develop high temperature strength through solid solution strengthening and precipitation strengthening from secondary phase precipitates such as gamma prime and carbides. Oxidation or corrosion resistance is provided by elements such as aluminium and chromium. Superalloys are often cast as a single crystal—while grain boundaries may provide strength at low temperatu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
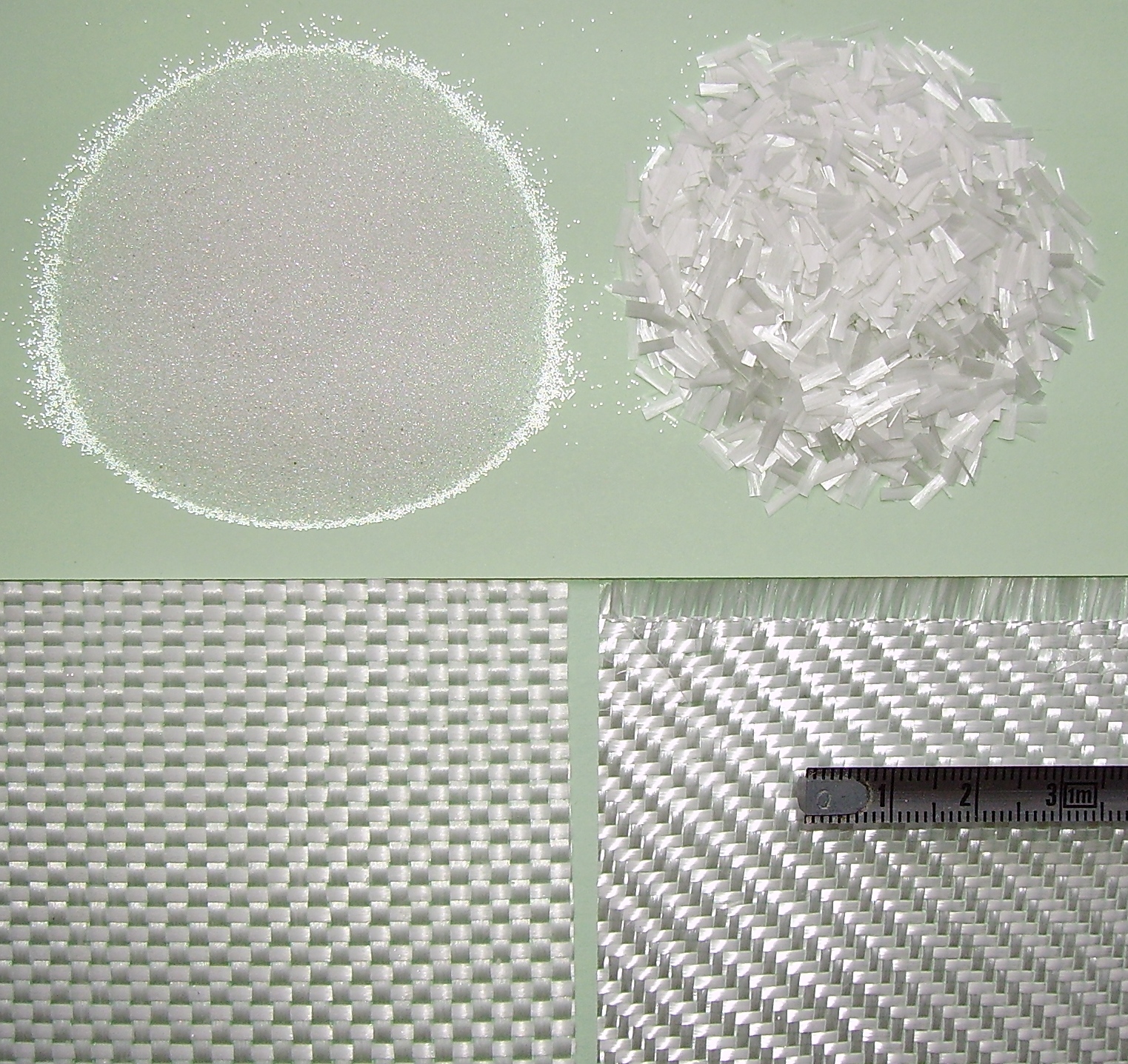
Fiberglass
Fiberglass (American English) or fibreglass ( Commonwealth English) is a common type of fiber-reinforced plastic using glass fiber. The fibers may be randomly arranged, flattened into a sheet called a chopped strand mat, or woven into glass cloth. The plastic matrix may be a thermoset polymer matrix—most often based on thermosetting polymers such as epoxy, polyester resin, or vinyl ester resin—or a thermoplastic. Cheaper and more flexible than carbon fiber, it is stronger than many metals by weight, non- magnetic, non-conductive, transparent to electromagnetic radiation, can be molded into complex shapes, and is chemically inert under many circumstances. Applications include aircraft, boats, automobiles, bath tubs and enclosures, swimming pools, hot tubs, septic tanks, water tanks, roofing, pipes, cladding, orthopedic casts, surfboards, and external door skins. Other common names for fiberglass are glass-reinforced plastic (GRP), glass-fiber reinforced plastic (GFR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicone
A silicone or polysiloxane is a polymer made up of siloxane (−R2Si−O−SiR2−, where R = organic group). They are typically colorless oils or rubber-like substances. Silicones are used in sealants, adhesives, lubricants, medicine, cooking utensils, thermal insulation, and electrical insulation. Some common forms include silicone oil, silicone grease, silicone rubber, silicone resin, and silicone caulk. Chemistry More precisely called polymerized siloxanes or polysiloxanes, silicones consist of an inorganic silicon–oxygen backbone chain (⋯−Si−O−Si−O−Si−O−⋯) with two organic groups attached to each silicon center. Commonly, the organic groups are methyl. The materials can be cyclic or polymeric. By varying the −Si−O− chain lengths, side groups, and crosslinking, silicones can be synthesized with a wide variety of properties and compositions. They can vary in consistency from liquid to gel to rubber to hard plastic. The most common si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicone Rubber
Silicone rubber is an elastomer (rubber-like material) composed of silicone—itself a polymer—containing silicon together with carbon, hydrogen, and oxygen. Silicone rubbers are widely used in industry, and there are multiple formulations. Silicone rubbers are often one- or two-part polymers, and may contain fillers to improve properties or reduce cost. Silicone rubber is generally non-reactive, stable, and resistant to extreme environments and temperatures from while still maintaining its useful properties. Due to these properties and its ease of manufacturing and shaping, silicone rubber can be found in a wide variety of products, including voltage line insulators; automotive applications; cooking, baking, and food storage products; apparel such as undergarments, sportswear, and footwear; electronics; medical devices and implants; and in home repair and hardware, in products such as silicone sealants. Curing In its uncured state, silicone rubber is a highly adhesive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |