|
CCPA (biochemistry)
2-Chloro-''N''6-cyclopentyladenosine (CCPA) is a specific receptor agonist for the Adenosine A1 receptor The adenosine A1 receptor is one member of the adenosine receptor group of G protein-coupled receptors with adenosine as endogenous ligand. Biochemistry A1 receptors are implicated in sleep promotion by inhibiting wake-promoting cholinergic ne .... It is similar to ''N''6-cyclopentyladenosine. References Nucleosides Purines Organochlorides Adenosine receptor agonists Cyclopentanes {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Agonist
An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an Receptor antagonist, antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Ancient Greek language, Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists Receptor (biochemistry), Receptors can be activated by either endogenous agonists (such as hormones and neurotransmitters) or exogenous agonists (such as medication, drugs), resulting in a biological response. A physiological agonism and ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine A1 Receptor
The adenosine A1 receptor is one member of the adenosine receptor group of G protein-coupled receptors with adenosine as endogenous ligand. Biochemistry A1 receptors are implicated in sleep promotion by inhibiting wake-promoting cholinergic neurons in the basal forebrain. A1 receptors are also present in smooth muscle throughout the vascular system. The adenosine A1 receptor has been found to be ubiquitous throughout the entire body. Signalling Activation of the adenosine A1 receptor by an agonist causes binding of Gi1/2/3 or Go protein. Binding of Gi1/2/3 causes an inhibition of adenylate cyclase and, therefore, a decrease in the cAMP concentration. An increase of the inositol triphosphate/diacylglycerol concentration is caused by an activation of phospholipase C, whereas the elevated levels of arachidonic acid are mediated by DAG lipase, which cleaves DAG to form arachidonic acid. Several types of potassium channels are activated but N-, P-, and Q-type calcium channels ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N6-Cyclopentyladenosine
''N''6-Cyclopentyladenosine (CPA) is a drug which acts as a selective adenosine A1 receptor agonist. It has mainly cardiovascular effects with only subtle alterations of behavior. CPA is widely used in scientific research into the adenosine receptor The adenosine receptors (or P1 receptors) are a class of purinergic G protein-coupled receptors with adenosine as the endogenous ligand. There are four known types of adenosine receptors in humans: A1, A2A, A2B and A3; each is encoded by a di ...s and has been used to derive a large family of derivatives. See also * ''N''6-Methyladenosine References Nucleosides Purines Adenosine receptor agonists Hydroxymethyl compounds {{cardiovascular-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleosides
Nucleosides are glycosylamines that can be thought of as nucleotides without a phosphate group. A nucleoside consists simply of a nucleobase (also termed a nitrogenous base) and a five-carbon sugar ( ribose or 2'-deoxyribose) whereas a nucleotide is composed of a nucleobase, a five-carbon sugar, and one or more phosphate groups. In a nucleoside, the anomeric carbon is linked through a glycosidic bond to the N9 of a purine or the N1 of a pyrimidine. Nucleotides are the molecular building-blocks of DNA and RNA. List of nucleosides and corresponding nucleobases The reason for 2 symbols, shorter and longer, is that the shorter ones are better for contexts where explicit disambiguation is superfluous (because context disambiguates) and the longer ones are for contexts where explicit disambiguation is judged to be needed or wise. For example, when discussing long nucleobase sequences in genomes, the CATG symbol system is much preferable to the Cyt-Ade-Thy-Gua symbol system (s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
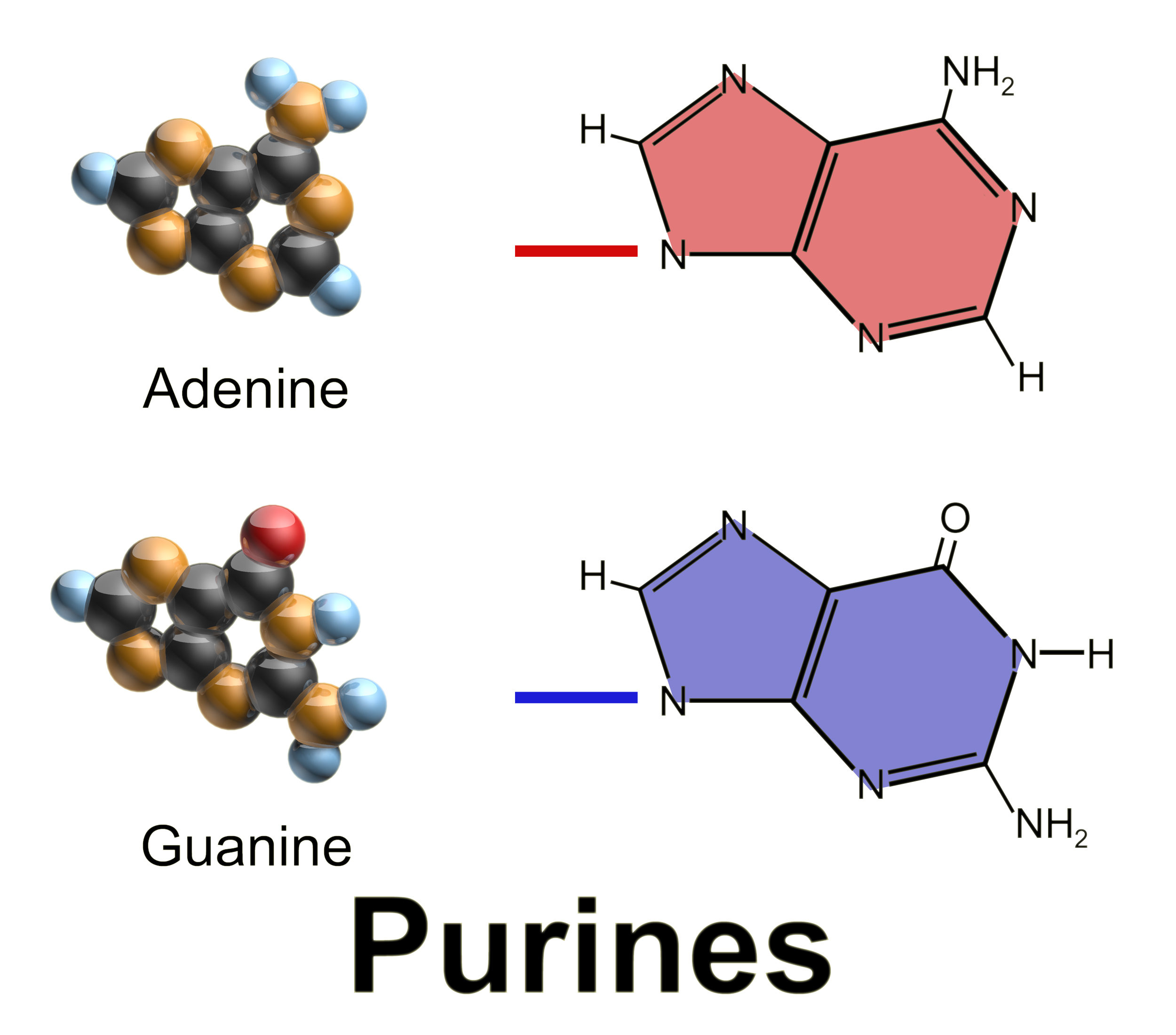
Purines
Purine is a heterocyclic aromatic organic compound that consists of two rings (pyrimidine and imidazole) fused together. It is water-soluble. Purine also gives its name to the wider class of molecules, purines, which include substituted purines and their tautomers. They are the most widely occurring nitrogen-containing heterocycles in nature. Dietary sources Purines are found in high concentration in meat and meat products, especially internal organs such as liver and kidney. In general, plant-based diets are low in purines. High-purine plants and algae include some legumes (lentils and black eye peas) and spirulina. Examples of high-purine sources include: sweetbreads, anchovies, sardines, liver, beef kidneys, brains, meat extracts (e.g., Oxo, Bovril), herring, mackerel, scallops, game meats, yeast (beer, yeast extract, nutritional yeast) and gravy. A moderate amount of purine is also contained in red meat, beef, pork, poultry, fish and seafood, asparagus, cauliflower ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organochlorides
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. Aliphatic organochlorides are often alkylating agents as chlorine can act as a leaving group, which can result in cellular damage. Natural occurrence Many organochlorine compounds have been iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine Receptor Agonists
Adenosine (symbol A) is an organic compound that occurs widely in nature in the form of diverse derivatives. The molecule consists of an adenine attached to a ribose via a β-N9-glycosidic bond. Adenosine is one of the four nucleoside building blocks of RNA (and its derivative deoxyadenosine is a building block of DNA), which are essential for all life. Its derivatives include the energy carriers adenosine mono-, di-, and triphosphate, also known as AMP/ADP/ATP. Cyclic adenosine monophosphate (cAMP) is pervasive in signal transduction. Adenosine is used as an intravenous medication for some cardiac arrhythmias. Adenosyl (abbreviated Ado or 5'-dAdo) is the chemical group formed by removal of the 5′-hydroxy (OH) group. It is found in adenosylcobalamin (an active form of vitamin B12) and as a radical in radical SAM enzymes. Medical uses Supraventricular tachycardia In individuals with supraventricular tachycardia (SVT), adenosine is used to help identify and convert the rh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |