|
Phytosterols
Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanols. More than 250 sterols and related compounds have been identified. Free phytosterols extracted from oils are insoluble in water, relatively insoluble in oil, and soluble in alcohols. Phytosterol-enriched foods and dietary supplements have been marketed for decades. Despite well-documented LDL cholesterol-lowering effects from long-term consumption of phytosterols, there is insufficient evidence for an effect on cardiovascular diseases, fasting blood sugar, glycated hemoglobin, or overall mortality rate. Structure They have a fused polycyclic structure and vary in carbon side chains and / or presence or absence of a double bond (saturation). They are divided into 4,4-dimethyl phytosterols, 4-monomethyl phytosterols, and 4-desmethyl phytosterols based on the location of methyl groups at the carbon-4 position. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils. Cholesterol is biosynthesis, biosynthesized by all animal Cell (biology)#Eukaryotic cells, cells and is an essential structural and cholesterol signaling, signaling component of animal cell membranes. In vertebrates, hepatocyte, hepatic cells typically produce the greatest amounts. In the brain, astrocytes produce cholesterol and transport it to neurons. It is absent among prokaryotes (bacteria and archaea), although there are some exceptions, such as ''Mycoplasma'', which require cholesterol for growth. Cholesterol also serves as a Precursor (chemistry), precursor for the biosynthesis of steroid hormones, bile acid and vitamin D. Elevated levels of cholesterol in the blood, especially when bound to low-density lipoprotein (LDL, often referred to as "bad cholesterol"), may increase the risk of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Campesterol
Campesterol is a phytosterol whose chemical structure is similar to that of cholesterol, and is one of the ingredients for E number E499. Natural occurrences Many vegetables, fruits, nuts, and seeds contain campesterol, but in low concentrations. Banana, pomegranate, pepper, coffee, grapefruit, cucumber, onion, oat, potato, and lemon grass (citronella) are few examples of common sources containing campesterol at roughly 1–7 mg/100 g of the edible portion. In contrast, canola and corn oils contain as much as 16–100 mg/100 g. Levels are variable and are influenced by geography and growing environment. In addition, different strains have different levels of plant sterols. A number of new genetic strains are currently being engineered with the goal of producing varieties high in campesterol and other plant sterols. It is also found in dandelion coffee. It is so named because it was first isolated from the rapeseed (''Brassica campestris''). Precursor o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LDL Cholesterol
Low-density lipoprotein (LDL) is one of the five major groups of lipoprotein that transport all fat molecules around the body in extracellular water. These groups, from least dense to most dense, are chylomicrons (aka ULDL by the overall density naming convention), very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL) and high-density lipoprotein (HDL). LDL delivers fat molecules to cells. LDL has been associated with the progression of atherosclerosis. Overview Lipoproteins transfer lipids (fats) around the body in the extracellular fluid, making fats available to body cells for receptor-mediated endocytosis. Lipoproteins are complex particles composed of multiple proteins, typically 80–100 proteins per particle (organized by a single apolipoprotein B for LDL and the larger particles). A single LDL particle is about 22–27.5 nanometers in diameter, typically transporting 3,000 to 6,000 fat molecules per particle and v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Campesterol
Campesterol is a phytosterol whose chemical structure is similar to that of cholesterol, and is one of the ingredients for E number E499. Natural occurrences Many vegetables, fruits, nuts, and seeds contain campesterol, but in low concentrations. Banana, pomegranate, pepper, coffee, grapefruit, cucumber, onion, oat, potato, and lemon grass (citronella) are few examples of common sources containing campesterol at roughly 1–7 mg/100 g of the edible portion. In contrast, canola and corn oils contain as much as 16–100 mg/100 g. Levels are variable and are influenced by geography and growing environment. In addition, different strains have different levels of plant sterols. A number of new genetic strains are currently being engineered with the goal of producing varieties high in campesterol and other plant sterols. It is also found in dandelion coffee. It is so named because it was first isolated from the rapeseed (''Brassica campestris''). Precursor o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stigmasterol
Stigmasterol – a plant sterol (''phytosterol'') – is among the most abundant of plant sterols, having a major function to maintain the structure and physiology of cell membranes. In the European Union, it is a food additive listed with E number E499, and may be used in food manufacturing to increase the phytosterol content, potentially lowering the levels of LDL cholesterol. Discovery Once called ''Wulzen factor'' in the mid-20th century, stigmasterol was discovered by the University of California physiologist Rosalind Wulzen (born 1886). Natural occurrences Stigmasterol is an unsaturated phytosterol occurring in the plant fats or oils of numerous plants, such as soybean, calabar bean, and rape seed, and in herbs used in herbalism practices, including the Chinese herbs '' Ophiopogon japonicus'' (Mai men dong), in '' Mirabilis jalapa''. Stigmasterol is a constituent of various vegetables, legumes, nuts, seeds, and unpasteurized milk. Pasteurization will inactiv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterol
A sterol is any organic compound with a Skeletal formula, skeleton closely related to Cholestanol, cholestan-3-ol. The simplest sterol is gonan-3-ol, which has a formula of , and is derived from that of gonane by replacement of a hydrogen atom on C3 position by a hydroxyl group. It is therefore an alcohol (chemistry), alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most Eukaryote, eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria (however likely with different functions). The most familiar type of animal sterol is cholesterol, which is vital to the structure of the cell membrane, and functions as a precursor to fat-soluble vitamins and steroid hormones. While technically alcohols, sterols are classified by biochemists as lipids (f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloartenol
Cycloartenol is an important triterpenoid often found in plants. It belongs to the sterol class of steroids. It is the starting point for the synthesis of almost all plant steroids, making them chemically distinct from the steroids of fungi and animals, which are, instead, produced from lanosterol. Synthesis The biosynthesis of cycloartenol starts from the triterpenoid squalene. It is the first precursor in the biosynthesis of other stanols and sterols, referred to as phytostanols and phytosterols in photosynthetic Photosynthesis ( ) is a Biological system, system of biological processes by which Photoautotrophism, photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical ener ... organisms and plants. The identities and distribution of phytostanols and phytosterols is characteristic of a plant species. References Sterols Triterpenes Cyclopropanes Pentacyclic compounds {{Alcohol- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
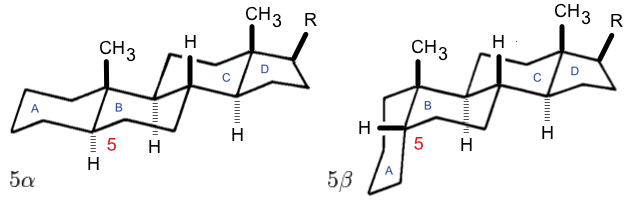
Steroid Numbering
A steroid is an organic compound with four fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in fungi, plants, and animals. All steroids are manufactured in cells from a sterol: cholesterol (animals), lanosterol ( opisthokonts), or cycloartenol (plants). All three of these molecules are produced via cyclization of the triterpene squalene. Structure The steroid nucleus ( core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in four fused rings: three six-member cyclohexane rings (rings A, B and C in the first illustration) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytosteroid
Phytosteroids, also known as plant steroids, are natural product, naturally occurring steroids that are found in plants. Examples include digoxin, digitoxin, diosgenin, and guggulsterone, as well as phytosterols like β-sitosterol. Industrial use Steroid pharmaceuticals that are identical or similar to human steroid hormones are very widely used in medicine. However, the four-ring structure of a steroid is quite expensive to replicate using direct synthetic methods. In 1938–1940, American chemist Russell Earl Marker developed the process known as Marker degradation, which converts diosgenin from Mexican ''Dioscorea'' yams into 16-dehydropregnenolone acetate, which has a four-ring structure and can be used to synthesize commonly used steroid hormones. Marker's process reduced the price of progesterone from $80/gram in early 1944 to $2/gram in 1951. Also in 1940, American chemist Percy Lavon Julian discovered a process to convert a much more abundant phytosteroid -- stigmast ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brassicasterol
Brassicasterol (24-methyl cholest-5,22-dien-3β-ol) is a 28-carbon sterol synthesised by several unicellular algae (phytoplankton) and some terrestrial plants, like rape. This compound has frequently been used as a biomarker for the presence of (marine) algal matter in the environment, and is one of the ingredients in stigmasterol-rich plant sterols (Number E499 in the European numbering system). There is some evidence to suggest that it may also be a relevant additional biomarker in Alzheimer's disease. Chemical properties Solubility Brassicasterol has a low water solubility and, as a consequence, a high octanol-water partition coefficient. This means that, in most environmental systems, brassicasterol will be associated with the solid phase. Degradation In anaerobic sediments and soils, brassicasterol is stable for many hundreds of years, enabling it to be used as an indicator of past algal production (see below). Chemical analysis Since the molecule has a hydroxyl (-OH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |