|
Cycloartenol
Cycloartenol is an important triterpenoid often found in plants. It belongs to the sterol class of steroids. It is the starting point for the synthesis of almost all plant steroids, making them chemically distinct from the steroids of fungi and animals, which are, instead, produced from lanosterol. Synthesis The biosynthesis of cycloartenol starts from the triterpenoid squalene. It is the first precursor in the biosynthesis of other stanols and sterols, referred to as phytostanols and phytosterols in photosynthetic Photosynthesis ( ) is a Biological system, system of biological processes by which Photoautotrophism, photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical ener ... organisms and plants. The identities and distribution of phytostanols and phytosterols is characteristic of a plant species. References Sterols Triterpenes Cyclopropanes Pentacyclic compounds {{Alcohol- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
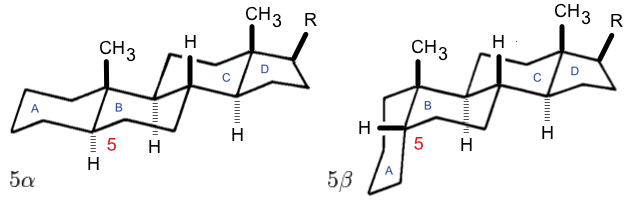
Steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signal transduction, signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in Fungus, fungi, plants, and animals. All steroids are manufactured in cells from a sterols, sterol: Cholesterol, cholesterol (animals), lanosterol (opisthokonts), or cycloartenol (plants). All three of these molecules are produced via Cyclic compound, cyclization of the triterpene squalene. Structure The steroid nucleus (parent structure, core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, having a role in maintaining health of the lens. Lanosterol is the precursor to cholesterol. Biosynthesis The biosynthesis of lanosterol has been intensively investigated. Elaboration of lanosterol under enzyme catalysis leads to other steroids. 14-Demethylation of lanosterol by CYP51 eventually yields cholesterol. Research as an eye drop supplement As a molecule naturally enriched in the eye lens, lanosterol is a component involved in maintenance of lens clarity. Its proposed mechanism of action is to inhibit the aggregation of crystallin proteins, which contribute to the clouding of vision by forming cataracts. Lanosterol is under research for its potential as a therapeutic additive in eye drops to inhibit the aggregation of crysta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpenoid
Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' often are used i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterol
A sterol is any organic compound with a Skeletal formula, skeleton closely related to Cholestanol, cholestan-3-ol. The simplest sterol is gonan-3-ol, which has a formula of , and is derived from that of gonane by replacement of a hydrogen atom on C3 position by a hydroxyl group. It is therefore an alcohol (chemistry), alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most Eukaryote, eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria (however likely with different functions). The most familiar type of animal sterol is cholesterol, which is vital to the structure of the cell membrane, and functions as a precursor to fat-soluble vitamins and steroid hormones. While technically alcohols, sterols are classified by biochemists as lipids (f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-Catalysis, catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve as enzyme substrate (chemistry), substrates, with conversion by the living organism either into simpler or more complex Product (chemistry), products. Examples of biosynthetic pathways include those for the production of amino acids, lipid membrane components, and nucleotides, but also for the production of all classes of biological macromolecules, and of acetyl-coenzyme A, adenosine triphosphate, nicotinamide adenine dinucleotide and other key intermediate and transactional molecules needed for metabolism. Thus, in biosynthesis, any of an array of Chemical compound, compounds, from simple to complex, are converted into other compounds, and so it includes both the catabolism and anabolism (building up and breaking down) of comple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Squalene
Squalene is an organic compound. It is a triterpene with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as '' Squalus'' is a genus of sharks). An estimated 12% of bodily squalene in humans is found in sebum. Squalene has a role in topical skin lubrication and protection. Most plants, fungi, and animals produce squalene as biochemical precursor in sterol biosynthesis, including cholesterol and steroid hormones in the human body. It is also an intermediate in the biosynthesis of hopanoids in many bacteria. Squalene is an important ingredient in some vaccine adjuvants: The Novartis and GlaxoSmithKline adjuvants are called MF59 and AS03, respectively. Role in triterpenoid synthesis Squalene is a biochemical precursor to both steroids and hopanoids. For sterols, the squalene conversion begins with oxidation (via squalene monooxygenase) of one of its terminal double bonds, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stanol
A sterol is any organic compound with a Skeletal formula, skeleton closely related to Cholestanol, cholestan-3-ol. The simplest sterol is gonan-3-ol, which has a formula of , and is derived from that of gonane by replacement of a hydrogen atom on C3 position by a hydroxyl group. It is therefore an alcohol (chemistry), alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most Eukaryote, eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria (however likely with different functions). The most familiar type of animal sterol is cholesterol, which is vital to the structure of the cell membrane, and functions as a precursor to fat-soluble vitamins and steroid hormones. While technically alcohols, sterols are classified by biochemists as lipids (f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytosterol
Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanol ester, stanols. More than 250 sterols and related compounds have been identified. Free phytosterols extracted from oils are insoluble in water, relatively insoluble in oil, and soluble in alcohols. Phytosterol-enriched foods and dietary supplements have been marketed for decades. Despite well-documented LDL cholesterol-lowering effects from long-term consumption of phytosterols, there is insufficient evidence for an effect on cardiovascular diseases, fasting blood sugar, glycated hemoglobin, or overall mortality rate. Structure They have a fused polycyclic structure and vary in carbon side chains and / or presence or absence of a double bond (saturation). They are divided into 4,4-dimethyl phytosterols, 4-monomethyl phytosterols, and 4-desmethyl phytosterols based on the location of methyl groups at the carbon-4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabolism. ''Photosynthesis'' usually refers to oxygenic photosynthesis, a process that produces oxygen. Photosynthetic organisms store the chemical energy so produced within intracellular organic compounds (compounds containing carbon) like sugars, glycogen, cellulose and starches. To use this stored chemical energy, an organism's cells metabolize the organic compounds through cellular respiration. Photosynthesis plays a critical role in producing and maintaining the oxygen content of the Earth's atmosphere, and it supplies most of the biological energy necessary for complex life on Earth. Some bacteria also perform anoxygenic photosynthesis, which uses bacteriochlorophyll to split hydrogen sulfide as a reductant instead of water, p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sterols
A sterol is any organic compound with a Skeletal formula, skeleton closely related to Cholestanol, cholestan-3-ol. The simplest sterol is gonan-3-ol, which has a formula of , and is derived from that of gonane by replacement of a hydrogen atom on C3 position by a hydroxyl group. It is therefore an alcohol (chemistry), alcohol of gonane. More generally, any compounds that contain the gonane structure, additional functional groups, and/or modified ring systems derived from gonane are called steroids. Therefore, sterols are a subgroup of the steroids. They occur naturally in most Eukaryote, eukaryotes, including plants, animals, and fungi, and can also be produced by some bacteria (however likely with different functions). The most familiar type of animal sterol is cholesterol, which is vital to the structure of the cell membrane, and functions as a precursor to fat-soluble vitamins and steroid hormones. While technically alcohols, sterols are classified by biochemists as lipids (f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropanes
Cyclopropanes are a family of organic compounds containing the cyclopropyl group. The parent is cyclopropane (). Synthesis and reactions Most cyclopropanes are not prepared from the parent cyclopropane, which is somewhat inert. Instead, yclopropyl groups are often prepared by cyclization of 1,3-difunctional alkanes. An example of the former, cyclopropyl cyanide is prepared by the reaction of 4- chlorobutyronitrile with a strong base. Phenylcyclopropane is produced analogously from the 1,3-dibromide. A second major route to cyclopropanes entails addition of methylene (or its substituted derivatives) to an alkene, a process called cyclopropanation. Substituted cyclopropanes undergo the reactions associated with the cyclopropyl ring or the substituents. Vinylcyclopropanes are a special case as they undergo vinylcyclopropane rearrangement. Simple substituted cyclopropanes * Chlorocyclopropane * Cyclopropane carboxylic acid * Cyclopropyl amine * Cyclopropyl cyanide * Cycloprop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |