|
Lanosterol 14α-demethylase Inhibitors
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, having a role in maintaining health of the lens. Lanosterol is the precursor to cholesterol. Biosynthesis The biosynthesis of lanosterol has been intensively investigated. Elaboration of lanosterol under enzyme catalysis leads to other steroids. 14-Demethylation of lanosterol by CYP51 eventually yields cholesterol. Research as an eye drop supplement As a molecule naturally enriched in the eye lens, lanosterol is a component involved in maintenance of lens clarity. Its proposed mechanism of action is to inhibit the aggregation of crystallin proteins, which contribute to the clouding of vision by forming cataracts. Lanosterol is under research for its potential as a therapeutic additive in eye drops to inhibit the aggregation of crystallin p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetracyclic
Tetracyclics are cyclic compound, cyclic chemical compounds that contain four fused ring (chemistry), rings of atoms, for example, Tröger's base. Some tricyclic compounds having three fused and one tethered ring (connected to main nucleus by a single bond) can also classified as tetracyclic, for example, ciclazindol. Tetracyclic compounds have various pharmaceutical drug, pharmaceutical uses, such as: *tetracycline antibiotics **Doxycycline **Tigecycline **Omadacycline **Eravacycline *tetracyclic antidepressants **Benzoctamine **Loxapine **Mazindol **Mianserin **Mirtazapine See also * Tricyclic * Heterocyclic References {{Tetracyclics Tetracyclic compounds, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Demethylation
Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen atoms. The counterpart of demethylation is methylation. In biochemistry : Demethylation is relevant to epigenetics. Demethylation of DNA is catalyst, catalyzed by demethylases. These enzymes oxidize N-methyl groups, which occur in histones, in lysine derivatives, and in some forms of DNA. :R2N-CH3 + O → R2N-H + CH2O One family of such oxidative enzymes is the cytochrome P450. Alpha-ketoglutarate-dependent hydroxylases are also active for demethylation of DNA, operating by a similar stoichiometry. These reactions, which proceed via hydroxylation, exploit the slightly weakened Carbon–hydrogen bond, C-H bonds of methylamines and methyl ethers. Demethylation of some sterols are steps in the biosynthesis of testosterone and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cucurbitacin
Cucurbitacins are a class of biochemistry, biochemical compounds that some plants – notably members of the pumpkin and gourd family, Cucurbitaceae – produce and which function as a defense against herbivores. Cucurbitacins and their derivative (chemistry), derivatives have also been found in many other plant families (including Brassicaceae, Scrophulariaceae, Begoniaceae, Elaeocarpaceae, Datiscaceae, Desfontainiaceae, Polemoniaceae, Primulaceae, Rubiaceae, Sterculiaceae, Rosaceae, and Thymelaeaceae), in some mushrooms (including ''Russula'' and ''Hebeloma'') and even in some marine mollusks. Cucurbitacins may be a taste deterrent in plants foraged by some animals and in some edible plants preferred by humans, such as cucumbers and zucchinis. In basic research, laboratory research, cucurbitacins have cytotoxic properties and are under study for their potential biological activity. Cucurbitacins are chemically classified as triterpenes, formally derived from cucurbitane, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tirucallol
Tirucallol is a naturally occurring tetracyclic triterpenoid alcohol found in various plant species, notably in the latex of '' Euphorbia lactea'' and the resin of ''Pistacia lentiscus'' (mastic tree). It is structurally related to other triterpenes and has been studied for its potential anti-inflammatory and antioxidant properties. Occurrence Tirucallol has been isolated from the latex of '' Euphorbia lactea'' and from the resin of ''Pistacia lentiscus'', commonly known as mastic gum. These natural sources have been traditionally used for their medicinal properties, and the presence of tirucallol contributes to their bioactivity. Structure and biological activity Tirucallol possesses a dammarane-type skeleton with a hydroxyl group at the 3β-position, classifying it as a tetracyclic triterpenoid alcohol. It is biosynthesized in plants via the mevalonate pathway, which leads to the formation of squalene and subsequent cyclization to various triterpenes. Research indicates that t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triterpene
Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the precursor to all steroids. Structures Triterpenes exist in a great variety of structures. Nearly 200 different skeletons have been identified. These skeletons may be broadly divided according to the number of rings present. In general pentacyclic structures (5 rings) tend to dominate. Squalene is biosynthesized through the head-to-head condensation of two farnesyl pyrophosphate units. This coupling converts a pair of C15 components into a C30 product. Squalene serves as precursor for the formation of many triterpenoids, including bacterial hopanoids and eukaryotic sterols. Triterpenoids By definition triterpenoids are triterpenes that possess heteroatoms, usually oxygen. The terms ''triterpene'' and ''triterpenoid'' often are used i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP51
In enzymology, a sterol 14-demethylase () is an enzyme of the cytochrome P450 (CYP) superfamily. It is any member of the CYP51 family. It catalysis, catalyzes a chemical reaction such as: :obtusifoliol + 3 O2 + 3 NADPH + 3 H+ \rightleftharpoons 4alpha-methyl-5alpha-ergosta-8,14,24(28)-trien-3beta-ol + formate + 3 NADP+ + 4 H2O The 4 substrate (biochemistry), substrates here are obtusifoliol, oxygen, O2, nicotinamide adenine dinucleotide phosphate, NADPH, and hydrogen ion, H+, whereas its 4 product (chemistry), products are 4alpha-methyl-5alpha-ergosta-8,14,24(28)-trien-3beta-ol, formate, nicotinamide adenine dinucleotide phosphate, NADP+, and water, H2O. Although the lanosterol 14α-demethylase is present in a wide variety of organisms, the enzyme is studied primarily in the context of fungi, where it plays an essential role in mediating membrane permeability. In fungi, CYP51 catalyzes the demethylation of lanosterol to create an important precursor that is eventually converted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cycloartenol
Cycloartenol is an important triterpenoid often found in plants. It belongs to the sterol class of steroids. It is the starting point for the synthesis of almost all plant steroids, making them chemically distinct from the steroids of fungi and animals, which are, instead, produced from lanosterol. Synthesis The biosynthesis of cycloartenol starts from the triterpenoid squalene. It is the first precursor in the biosynthesis of other stanols and sterols, referred to as phytostanols and phytosterols in photosynthetic Photosynthesis ( ) is a Biological system, system of biological processes by which Photoautotrophism, photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical ener ... organisms and plants. The identities and distribution of phytostanols and phytosterols is characteristic of a plant species. References Sterols Triterpenes Cyclopropanes Pentacyclic compounds {{Alcohol- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation. By definition, when a medication is administered intravenously, its bioavailability is 100%. However, when a medication is administered via routes other than intravenous, its bioavailability is lower due to intestinal epithelium absorption and first-pass metabolism. Thereby, mathematically, bioavailability equals the ratio of comparing the area under the plasma drug concentration curve versus time (AUC) for the extravascular formulation to the AUC for the intravascular formulation. AUC is used because AUC is proportional to the dose that has entered the systemic circulation. Bioavailability of a drug is an average value; to take population variability into account, deviation range is shown as ±. To ensure that the drug taker who has poor absorption is dosed appropriately, the bottom value of the deviation range is employed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eye Drop
Eye drops or eyedrops are liquid drops applied directly to the surface of the eye usually in small amounts such as a single drop or a few drops. Eye drops usually contain saline to match the salinity of the eye. Drops containing only saline and sometimes a lubricant are often used as artificial tears to treat dry eyes or simple eye irritation such as itching or redness. Eye drops may also contain one or more medications to treat a wide variety of eye diseases. Depending on the condition being treated, they may contain steroids, antihistamines, sympathomimetics, beta receptor blockers, parasympathomimetics, parasympatholytics, prostaglandins, nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, antifungals, or topical anesthetics. Eye drops have less of a risk of side effects than do oral medicines, and such risk can be minimized by occluding the lacrimal punctum (i.e. pressing on the inner corner of the eye) for a short while after instilling drops. Prior t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
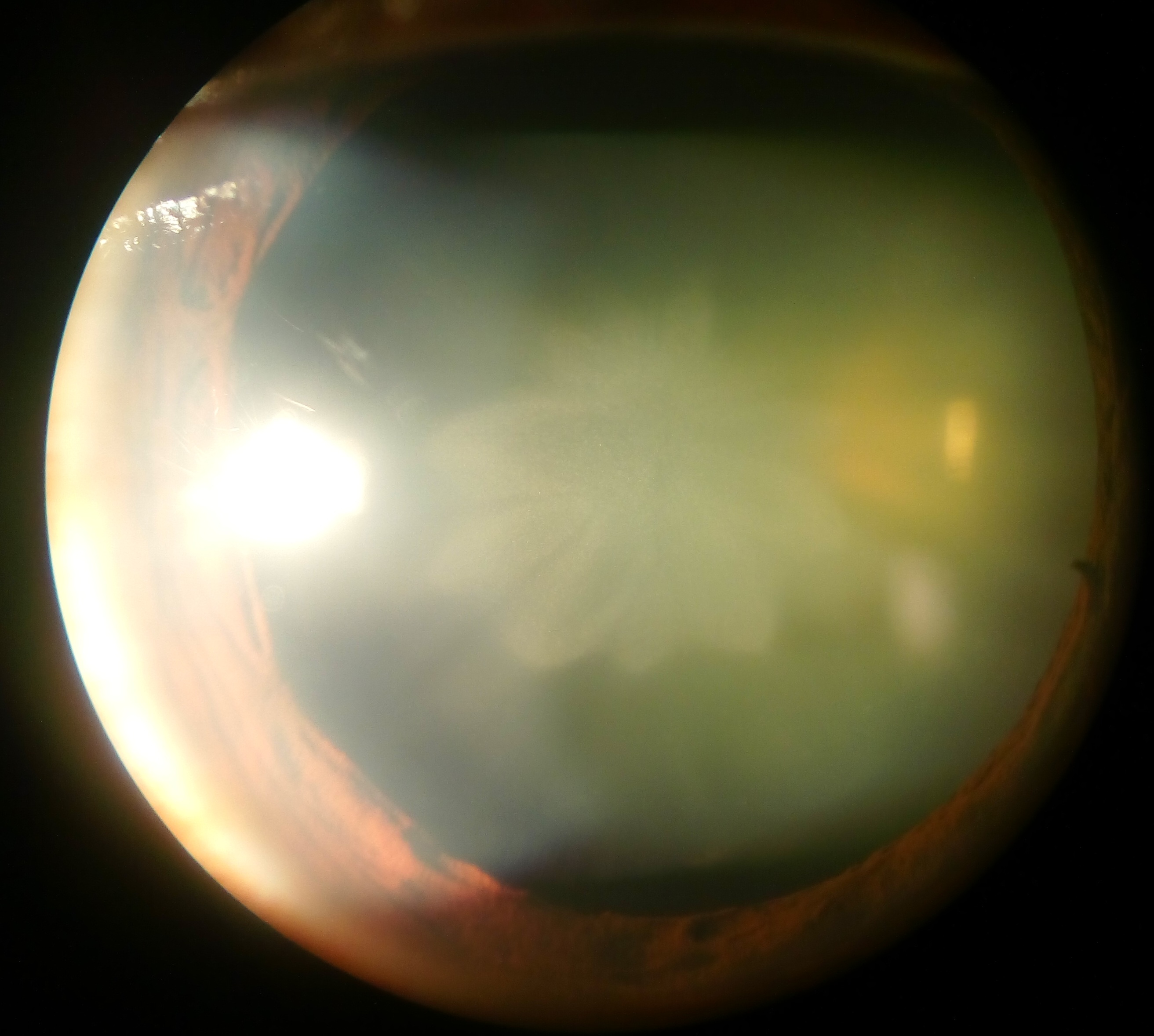
Cataract
A cataract is a cloudy area in the lens (anatomy), lens of the eye that leads to a visual impairment, decrease in vision of the eye. Cataracts often develop slowly and can affect one or both eyes. Symptoms may include faded colours, blurry or double vision, halos around light, trouble with bright lights, and Nyctalopia, difficulty seeing at night. This may result in trouble driving, reading, or recognizing faces. Poor vision caused by cataracts may also result in an increased risk of Falling (accident), falling and Depression (mood), depression. Cataracts cause 51% of all cases of blindness and 33% of visual impairment worldwide. Cataracts are most commonly due to senescence, aging but may also occur due to Trauma (medicine), trauma or radiation exposure, be congenital cataract, present from birth, or occur following eye surgery for other problems. Risk factors include diabetes mellitus, diabetes, longstanding use of corticosteroid medication, smoking tobacco, prolonged exposu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallin
In anatomy, a crystallin is a water-soluble structural protein found in the lens and the cornea of the eye accounting for the transparency of the structure. It has also been identified in other places such as the heart, and in aggressive breast cancer tumors. The physical origins of eye lens transparency and its relationship to cataract are an active area of research. Since it has been shown that lens injury may promote nerve regeneration, crystallin has been an area of neural research. So far, it has been demonstrated that crystallin β b2 (crybb2) may be a neurite-promoting factor. Function The main function of crystallins at least in the lens of the eye is probably to increase the refractive index while not obstructing light. However, this is not their only function. It has become clear that crystallins may have several metabolic and regulatory functions, both within the lens and in other parts of the body. More proteins containing βγ-crystallin domains have now been char ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |