|
Estranes
Estrane is a C18 steroid derivative, with a gonane core. ''Estrenes'' are estrane derivatives that contain a double bond, with an example being nandrolone. ''Estratrienes'' (estrins) are estrane derivatives that contain three double bonds, for instance estrin (compound), estrin (estra-1,3,5(10)-triene). A class of female sex hormones, estrogens, such as estradiol, estrone, and estriol are estratrienes. See also * Androstane * Pregnane References Estranes {{Steroid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nandrolone
Nandrolone, also known as 19-nortestosterone, is an endogenous androgen. It is also an anabolic steroid (AAS) which is medically used in the form of esters such as nandrolone decanoate (brand name Deca-Durabolin) and nandrolone phenylpropionate (brand name Durabolin). Nandrolone esters are used in the treatment of anemias, cachexia (Skeletal muscle, muscle wasting syndrome), osteoporosis, breast cancer, and for other indications. They are now used by oral administration or instead are given by intramuscular injection, injection into muscle or subcutaneous injection, fat. Side effects of nandrolone esters include symptoms of virilization, masculinization like acne, hirsutism, increased hair growth, and voice deepening, voice changes. They are synthetic compound, synthetic androgens and anabolic steroids and hence are agonists of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). Nandrolone has strong anabolic effects an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estratriene
Estrin (American English), or oestrin (British English), also known as estra-1,3,5(10)-triene, is an estrane steroid. It is dehydrogenated estrane with double bonds specifically at the C1, C3, and C5(10) positions. Estrin is a parent structure of the estrogen steroid hormones estradiol, estrone, and estriol, which have also been known as dihydroxyestrin, ketohydroxyestrin, and trihydroxyestrin, respectively. The estrin hydrocarbon itself possesses minimal estrogenic activity, as alcohol and/or keto substitutions at the C3 and C17 positions are critical for high binding affinity to the estrogen receptors. Estrin has been found to be on the order of 1,000-fold less potent than estradiol in inducing estrogenic responses ''in vitro''. In addition to estrin, estratrien-17β-ol, which lacks the 3-hydroxyl group of estradiol, and 3-hydroxyestratriene, which lacks the 17β-hydroxyl group of estradiol, both have measurable affinity for the estrogen receptor and are able to activate the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogen
Estrogen (also spelled oestrogen in British English; see spelling differences) is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal activity: estrone (E1), estradiol (E2), and estriol (E3). Estradiol, an estrane, is the most potent and prevalent. Another estrogen called estetrol (E4) is produced only during pregnancy. Estrogens are synthesized in all vertebrates and some insects. Quantitatively, estrogens circulate at lower levels than androgens in both men and women. While estrogen levels are significantly lower in males than in females, estrogens nevertheless have important physiological roles in males. Like all steroid hormones, estrogens readily diffuse across the cell membrane. Once inside the cell, they bind to and activate estrogen receptors (ERs) which in turn modulate the expression of many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estradiol
Estradiol (E2), also called oestrogen, oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of female reproductive cycles such as estrous and menstrual cycles. Estradiol is responsible for the development of female secondary sexual characteristics such as the breasts, widening of the hips and a female pattern of fat distribution. It is also important in the development and maintenance of female reproductive tissues such as the mammary glands, uterus and vagina during puberty, adulthood and pregnancy. It also has important effects in many other tissues including bone, fat, skin, liver, and the brain. Though estradiol levels in males are much lower than in females, estradiol has important roles in males as well. Apart from humans and other mammals, estradiol is also found in most vertebrates and crustaceans, insects, fish, and other animal species. Estradiol is produced within the follicles of the ovaries and in oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrone
Estrone (E1), also spelled oestrone, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estriol. Estrone, as well as the other estrogens, are synthesized from cholesterol and secreted mainly from the gonads, though they can also be formed from adrenal androgens in adipose tissue. Relative to estradiol, both estrone and estriol have far weaker activity as estrogens. Estrone can be converted into estradiol, and serves mainly as a precursor or metabolic intermediate of estradiol. It is both a precursor and metabolite of estradiol. In addition to its role as a natural hormone, estrone has been used as a medication, for instance in menopausal hormone therapy; for information on estrone as a medication, see the estrone (medication) article. Biological activity Estrone is an estrogen, specifically an agonist of the estrogen receptors ERα and ERβ. It is a far less potent estrogen than is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estriol
Estriol (E3), also spelled oestriol, is a steroid, a weak estrogen, and a minor female sex hormone. It is one of three major endogenous estrogens, the others being estradiol and estrone. Levels of estriol in women who are not pregnant are almost undetectable. However, during pregnancy, estriol is synthesized in very high quantities by the placenta and is the most produced estrogen in the body by far, although circulating levels of estriol are similar to those of other estrogens due to a relatively high rate of metabolism and excretion. Relative to estradiol, both estriol and estrone have far weaker activity as estrogens. In addition to its role as a natural hormone, estriol is used as a medication, for instance in menopausal hormone therapy; for information on estriol as a medication, see the estriol (medication) article. Biological activity Estriol is an estrogen, specifically an agonist of the estrogen receptors ERα and ERβ. It is a far less potent estrogen than is estra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration. Steroids have two principal biological functions: as important components of cell membranes that alter membrane fluidity; and as signal transduction, signaling molecules. Examples include the lipid cholesterol, sex hormones estradiol and testosterone, anabolic steroids, and the anti-inflammatory corticosteroid drug dexamethasone. Hundreds of steroids are found in Fungus, fungi, plants, and animals. All steroids are manufactured in cells from a sterols, sterol: Cholesterol, cholesterol (animals), lanosterol (opisthokonts), or cycloartenol (plants). All three of these molecules are produced via Cyclic compound, cyclization of the triterpene squalene. Structure The steroid nucleus (parent structure, core structure) is called gonane (cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon atoms, bonded in fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
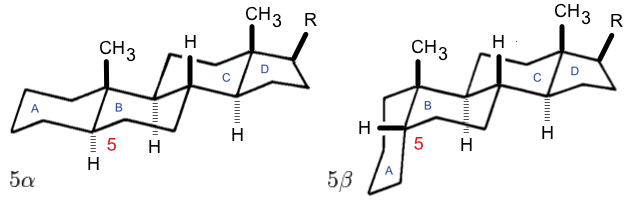
Gonane
Gonane (cyclopentanoperhydrophenanthrene) is a chemical compound with formula , whose structure consists of four hydrocarbon rings fused together: three cyclohexane units and one cyclopentane. It can also be viewed as the result of fusing a cyclopentane molecule with a fully hydrogenated molecule of phenanthrene, hence the more descriptive name "perhydrocyclopenta henanthrene". The non-systematic version of the above name is "cyclopentanoperhydrophenanthrene". It has no double bonds, that is, it is completely saturated and is considered the main structure of steroids, often referred to as the steroid nucleus. There are many forms of gonane, but only a few occur naturally in living organisms. Some common forms include 5α-gonane and 5β-gonane. Estrane, androstane, and pregnane are derivatives of gonane with additional methyl or ethyl groups attached to certain carbon positions. The term gonane is also used to describe a group of progestins that are similar to levonorgestr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist between two different elements: for example, in a carbonyl group between a carbon atom and an oxygen atom. Other common double bonds are found in azo compounds (N=N), imines (C=N), and sulfoxides (S=O). In a skeletal formula, a double bond is drawn as two parallel lines (=) between the two connected atoms; typographically, the equals sign is used for this. Double bonds were introduced in chemical notation by Russian chemist Alexander Butlerov. Double bonds involving carbon are stronger and shorter than single bonds. The bond order is two. Double bonds are also electron-rich, which makes them potentially more reactive in the presence of a strong electron acceptor (as in addition reactions of the halogens). File:Ethene structural.svg, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |