|
Dioxiranes
In chemistry, dioxirane (systematically named dioxacyclopropane, also known as methylene peroxide or peroxymethane) is an organic compound with formula . The molecule consists of a ring with one Methylene group, methylene and two oxygen atoms. It is of interest as the smallest cyclic organic peroxide, but otherwise it is of little practical value. Synthesis Dioxirane is highly unstable and the majority of studies of it have been computational chemistry, computational; it has been detected during the low temperature (–196 °C) reaction of ethylene and ozone, although even at these temperatures such a mixture can be explosive. Its formation is thought to be radical in nature, preceding via a Criegee intermediate. Microwave analysis has indicated C-H, C-O and O-O bond lengths of 1.090, 1.388 and 1.516 Å respectively. The very long and weak O-O bond (cf. hydrogen peroxide O-O = 1.47 Å) is the origin of its instability. Other dioxiranes Beyond the parent dioxirane, which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
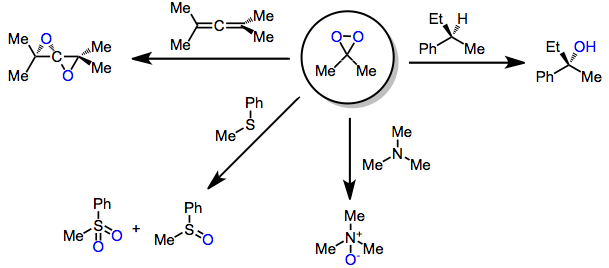
Dimethyldioxirane
Dimethyldioxirane (DMDO) is the organic compound with the formula . It is the dioxirane derived from acetone and can be viewed as the monomer of acetone peroxide. It is a powerful selective oxidizing agent that finds some use in organic synthesis. It is known only in the form of a dilute solution, usually in acetone, and hence the properties of the pure material are largely unknown. Synthesis DMDO is not commercially available because of chemical instability. DMDO can be prepared as dilute solutions (~0.1 M) by treatment of acetone with potassium peroxymonosulfate , usually in the form of Oxone (2KHSO5·KHSO4·K2SO4). : The preparation of DMDO is rather inefficient (typical yields < 3%) and typically only yields a relatively dilute solution in acetone (only up to approximately 0.1 M). This is tolerable as preparation uses inexpensive substances: acetone, sodium bicarbonate, and oxone. Cold solutions (−10 to −20 °C) of DMDO are stable for days. Decomposition is accelerat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dioxiranes
In chemistry, dioxirane (systematically named dioxacyclopropane, also known as methylene peroxide or peroxymethane) is an organic compound with formula . The molecule consists of a ring with one Methylene group, methylene and two oxygen atoms. It is of interest as the smallest cyclic organic peroxide, but otherwise it is of little practical value. Synthesis Dioxirane is highly unstable and the majority of studies of it have been computational chemistry, computational; it has been detected during the low temperature (–196 °C) reaction of ethylene and ozone, although even at these temperatures such a mixture can be explosive. Its formation is thought to be radical in nature, preceding via a Criegee intermediate. Microwave analysis has indicated C-H, C-O and O-O bond lengths of 1.090, 1.388 and 1.516 Å respectively. The very long and weak O-O bond (cf. hydrogen peroxide O-O = 1.47 Å) is the origin of its instability. Other dioxiranes Beyond the parent dioxirane, which i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimesityldioxirane
Dimesityldioxirane is a substituted dioxirane with two mesityl groups attached to the dioxirane carbon. It is a colorless crystalline substance stable in its solid state around -20 °C. Structure The molecule possesses approximately C2 symmetry and the mesityl groups are twisted by 54.2°. The bulky mesityl groups cause steric strain which is reduced by increase in R1–C–R2 angle from 117° in dioxirane to 119.2° in dimesityldioxirane. The mesityl groups also rotate about the single bond, consequently reducing steric repulsions further. Synthesis and Uses Dimesityldioxirane was first isolated at room temperature in pure as well as in solution form in 1994. It was synthesised by irradiation of its diazo derivative in trichlorofluoromethane at 183K to form , followed by oxidation. The synthesis had a yield of 50%. It can mainly be used as an oxidizing agent but is less oxidizing than other dioxiranes due to its stability. See also * Difluorodioxirane *Dimethyldioxir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Difluorodioxirane
Difluorodioxirane (CF2O2) is a rare, stable member of the dioxirane family, known for a single oxygen-oxygen bond (O-O). Unlike most dioxiranes that decompose quickly, difluorodioxirane is surprisingly stable at room temperature, making it potentially useful for further research and applications. Synthesis Difluorodioxirane was first synthesised by Russo and DesMarteau in 1993 by treating fluorocarbonyl hypofluorite (FCOOF) with X2 (= F2, Cl2 or ClF) over pelletized CsF in a flow system. It also likely exists as a possible intermediate in reactions involving other fluorine-containing compounds. Properties Unlike most dioxiranes that decompose quickly, difluorodioxirane is surprisingly stable at room temperature due to the stabilising interacton of two fluorine atoms with the ring. This effect makes the O-O bond less reactive and more stable compared to other dioxiranes. The central F–C–F angle is 109°, approximately a tetrahedral angle. Difluorodioxirane is known fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shi Epoxidation
The Shi epoxidation is a chemical reaction described as the asymmetric epoxidation of alkenes with oxone (potassium peroxymonosulfate) and a fructose-derived catalyst (1). This reaction is thought to proceed via a dioxirane intermediate, generated from the catalyst ketone by oxone (potassium peroxymonosulfate). The addition of the sulfate group by the oxone facilitates the formation of the dioxirane by acting as a good leaving group during ring closure. It is notable for its use of a non-metal catalyst and represents an early example of organocatalysis. History The reaction was first reported by Yian Shi (史一安, pinyin: Shǐ Yī-ān) is derived from D-fructose and has a stereogenic center close to the reacting center (ketone)- the rigid six-membered ring structure of the catalyst and adjacent quaternary ring group minimizes epimerization of this stereocenter. Oxidation by the active dioxirane catalyst takes place from the si-face, due to steric hindrance of the opposing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Ozone
Cyclic ozone is a theoretically predicted form of ozone. Like ordinary ozone (O3), it would have three oxygen atoms. It would differ from ordinary ozone in how those three oxygen atoms are arranged. In ordinary ozone, the atoms are arranged in a bent line; in cyclic ozone, they would form an equilateral triangle. Some of the properties of cyclic ozone have been predicted theoretically. It should have more energy than ordinary ozone. There is evidence that tiny quantities of cyclic ozone exist at the surface of magnesium oxide crystals in air. Cyclic ozone has not been made in bulk, although at least one researcher has attempted to do so using lasers. Another possibility to stabilize this form of oxygen is to produce it inside confined spaces, e.g., fullerene. It has been speculated that, if cyclic ozone could be made in bulk, and if it proved to have good stability properties, it could be added to liquid oxygen to improve the specific impulse Specific impulse (usually ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,3-Dioxetane
1,3-Dioxetane (1,3-dioxacyclobutane) is a heterocyclic organic compound with formula C2O2H4, whose backbone is a four-member ring of alternating oxygen and carbon atoms. It can be viewed as a dimer of formaldehyde Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as ... (COH2). Derivatives of 1,3-dioxetane are rarely encountered as intermediates in the literature. Usually, they are prepared via +2cycloadditions of two carbonyl compounds. Molecular orbital theory calculations suggest that they should be more stable than the 1,2-isomers, which are more intensively studied. See also * 1,2-Dioxetane References Dioxetanes {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Dioxetane
The chemical substance 1,2-dioxetane (systematically named 1,2-dioxacyclobutane, also known as ethylene peroxide or peroxyethane) is a heterocyclic compound, heterocyclic, organic compound with formula , containing a Ring (chemistry), ring of two adjacent oxygen atoms and two adjacent carbon atoms. It is therefore an organic peroxide, and can be viewed as a Dimer (chemistry), dimer of formaldehyde (). Luminescence Chemiluminescence was first observed with lophine (triphenylimidazole). When in basic solution, this compound converts to the imidazolate, which reacts with oxygen to eventually give the 1,2-dioxetane. Fragmentation of the dioxetane gives the excited state of an anionic diamide. In the 1960s, 1,2-dioxetane were demonstrated as intermediates in the reactions responsible for the bioluminescence in firefly, fireflies, glow-worms, and other luminescent creatures. The luminescence of glowsticks and luminescent bangles and necklaces involves 1,2-dioxetanedione (CO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Oxide
Ethylene oxide is an organic compound with the chemical formula, formula . It is a cyclic ether and the simplest epoxide: a three-membered ring (chemistry), ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of a silver catalyst. The reactivity that is responsible for many of ethylene oxide's hazards also makes it useful. Although too dangerous for direct household use and generally unfamiliar to consumers, ethylene oxide is used for making many consumer products as well as non-consumer chemicals and intermediates. These products include detergents, thickeners, solvents, plastics, and various organic chemicals such as ethylen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxaziridine
An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon. In their largest industrial application, oxaziridines are intermediates in the production of hydrazine. Oxaziridine derivatives are also used as specialized organic chemistry reagents for a variety of enantioselective oxidations and aminations. Oxaziridines also serve as precursors to nitrones and participate in +2cycloadditions with various heterocumulenes to form substituted five-membered heterocycles. Some oxaziridines also have the property of a high barrier to inversion of the nitrogen, allowing for the possibility of chirality at the nitrogen center. History Oxaziridine derivatives were first reported in the mid-1950s by Emmons and subsequently by Krimm and Horner and Jürgens. All noted that oxaziridine underwent unusual reactions, with both nitrogen and oxygen acting contrary to their usual polarity. The peroxide process for the industria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |