Oxaziridine on:
[Wikipedia]
[Google]
[Amazon]
An oxaziridine is an


 ''N''-sulfonyloxaziridines oxidize enolates to acyloins with high chiral induction, better than (e.g.) MoOPH. Chiral induction has been demonstrated with many chiral auxiliaries, including SAMP and RAMP; high yield (77–91%) and ''dr'' (95:5 – 99:1) are reported with the Evans' chiral oxazolidinones.
''N''-sulfonyloxaziridines oxidize enolates to acyloins with high chiral induction, better than (e.g.) MoOPH. Chiral induction has been demonstrated with many chiral auxiliaries, including SAMP and RAMP; high yield (77–91%) and ''dr'' (95:5 – 99:1) are reported with the Evans' chiral oxazolidinones.
 Extensive work has been reported on asymmetric hydroxylation of prochiral enolates with camphorsulfonyloxaziridine derivatives, achieving moderate to high
Extensive work has been reported on asymmetric hydroxylation of prochiral enolates with camphorsulfonyloxaziridine derivatives, achieving moderate to high  The selectivity of some hydroxylations may be drastically improved in some cases with the addition of coordinating groups alpha to the oxaziridine ring as oxaziridines 3b and 3c. In these instances it is proposed that the reaction proceeds through a closed transition state where the metal oxyanion is stabilized by
The selectivity of some hydroxylations may be drastically improved in some cases with the addition of coordinating groups alpha to the oxaziridine ring as oxaziridines 3b and 3c. In these instances it is proposed that the reaction proceeds through a closed transition state where the metal oxyanion is stabilized by  α-Hydroxylation with oxaziridines has been widely implemented in total synthesis. It is a key step in both the Holton Taxol total synthesis and the Wender Taxol total synthesis.
α-Hydroxylation with oxaziridines has been widely implemented in total synthesis. It is a key step in both the Holton Taxol total synthesis and the Wender Taxol total synthesis.
 Further investigation into these reactions may be required before levels of enantiometic excess become practical for large scale synthesis.
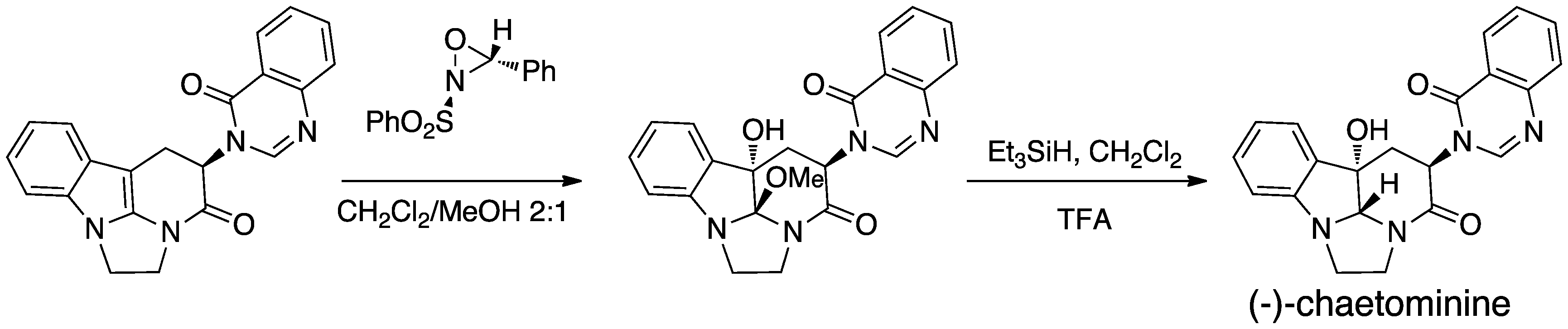
Oxaziridines can also form highly acid-sensitive epoxides, as in the following conclusion to a (−)-chaetominine synthesis:
Further investigation into these reactions may be required before levels of enantiometic excess become practical for large scale synthesis.
Oxaziridines can also form highly acid-sensitive epoxides, as in the following conclusion to a (−)-chaetominine synthesis:



 Aubé takes advantage of this rearrangement as the key step in his synthesis of (+)-
Aubé takes advantage of this rearrangement as the key step in his synthesis of (+)-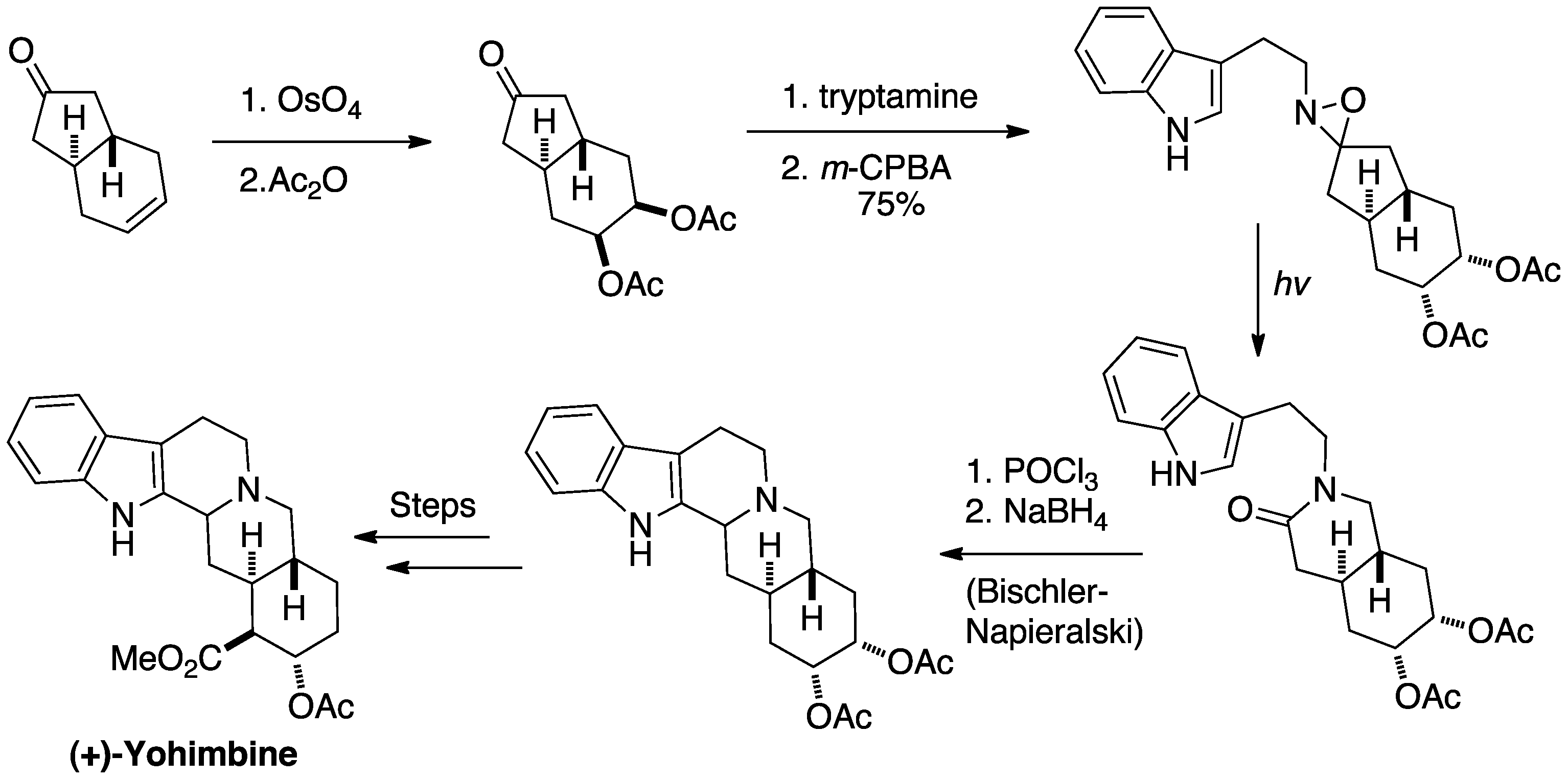 It is also notable that oxaziridines will thermally rearrange to nitrones. Cis-trans selectivity of the resulting nitrone is poor, however, yields are good to excellent. It is thought that some oxaziridines racemize over time through a nitrone intermediate.
It is also notable that oxaziridines will thermally rearrange to nitrones. Cis-trans selectivity of the resulting nitrone is poor, however, yields are good to excellent. It is thought that some oxaziridines racemize over time through a nitrone intermediate.


 Oxidation of chiral imines and oxidation of imines with chiral peracids may yield enantiopure oxaziridines.
Oxidation of chiral imines and oxidation of imines with chiral peracids may yield enantiopure oxaziridines.

organic molecule
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-cont ...
that features a three-membered heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
containing oxygen, nitrogen, and carbon. In their largest industrial application, oxaziridines are intermediates in the production of hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
. Oxaziridine derivatives are also used as specialized organic chemistry reagents for a variety of enantioselective
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation o ...
oxidations and aminations. Oxaziridines also serve as precursors to nitrones and participate in +2cycloadditions with various heterocumulenes to form substituted five-membered heterocycles.
Some oxaziridines also have the property of a high barrier to inversion of the nitrogen, allowing for the possibility of chirality at the nitrogen center.
History
Oxaziridine derivatives were first reported in the mid-1950s by Emmons and subsequently by Krimm and Horner and Jürgens. All noted that oxaziridine underwent unusual reactions, with both nitrogen and oxygen acting contrary to their usual polarity. Theperoxide process
The peroxide process is a method for the industrial production of hydrazine.
In this process hydrogen peroxide is used as an oxidant instead of sodium hypochlorite, which is traditionally used to generate hydrazine. The main advantage of the pero ...
for the industrial production of hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
through the oxidation of ammonia
Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammonia is a colourless gas with a distinctive pu ...
with hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
in the presence of ketones was developed in the early 1970s.. ..
In the late 1970s and early 1980s Franklin A. Davis synthesized the first ''N''-sulfonyloxaziridines, which act exclusively as oxygen transfer reagents, and are the most predominantly used class of oxaziridines today.
Chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
camphorsulfonyloxaziridines proved useful in the syntheses of complex products, such as taxol which is marketed as a chemotherapy agent. Both the Holton Taxol total synthesis and the Wender Taxol total synthesis feature asymmetric α-hydroxylation with camphorsulfonyloxaziridine.
Additionally, Forsyth implemented the transformation in his synthesis of the C3-C14 (substituted 1,7-Dioxaspiro .5ndec-3-ene) System of okadaic acid
Okadaic acid, C44H68O13, is a toxin produced by several species of dinoflagellates. It is known to accumulate in both marine sponges and shellfish. One of the primary causes of diarrhetic shellfish poisoning, okadaic acid is a potent inhibitor of ...
.
Structure and reactivity
Whereas oxygen and nitrogen typically act asnucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
s due to their high electronegativity
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
, oxaziridines allow for electrophilic
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carr ...
transfer of either heteroatom. The unusual reactivity occurs because the central three-membered ring has high strain, producing a relatively weak N-O bond.
Some oxaziridines inhibit nitrogen inversion at room temperature, with an energy barrier of 100 to 130 kJ/mol. Enantiopure oxaziridines where stereochemistry is entirely due to configurationally stable nitrogen are reported.
Nucleophiles tend to attack at the aziridine nitrogen when the nitrogen substituent is small (R1= H), and at the oxygen atom when the nitrogen substituent has greater steric bulk.

Hydrazine production
Oxaziridines are intermediates in theperoxide process
The peroxide process is a method for the industrial production of hydrazine.
In this process hydrogen peroxide is used as an oxidant instead of sodium hypochlorite, which is traditionally used to generate hydrazine. The main advantage of the pero ...
for hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
. Many millions of kilograms of hydrazine are produced annually by this method that involves a step wherein ammonia is oxidized in the presence of methyl ethyl ketone
Butanone, also known as methyl ethyl ketone (MEK) or ethyl methyl ketone, is an organic compound with the formula CH3C(O)CH2CH3. This colorless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large s ...
to give the oxaziridine:Jean-Pierre Schirmann, Paul Bourdauducq "Hydrazine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. .
:Me(Et)C=O + NH3 + H2O2 → Me(Et)CONH + 2H2O
In subsequent steps the oxaziridine is converted to the hydrazone, which is the immediate in the way to hydrazine:
:Me(Et)CONH + NH3 → Me(Et)C=NNH2 + H2O
Oxygen transfer
α-Hydroxylation of enolates
 Extensive work has been reported on asymmetric hydroxylation of prochiral enolates with camphorsulfonyloxaziridine derivatives, achieving moderate to high
Extensive work has been reported on asymmetric hydroxylation of prochiral enolates with camphorsulfonyloxaziridine derivatives, achieving moderate to high enantiomeric excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a sing ...
. The commonly accepted transition state is open
Open or OPEN may refer to:
Music
* Open (band), Australian pop/rock band
* The Open (band), English indie rock band
* ''Open'' (Blues Image album), 1969
* ''Open'' (Gerd Dudek, Buschi Niebergall, and Edward Vesala album), 1979
* ''Open'' (Go ...
, wherefore the steric bulk of R1 determines the face of approach.
 The selectivity of some hydroxylations may be drastically improved in some cases with the addition of coordinating groups alpha to the oxaziridine ring as oxaziridines 3b and 3c. In these instances it is proposed that the reaction proceeds through a closed transition state where the metal oxyanion is stabilized by
The selectivity of some hydroxylations may be drastically improved in some cases with the addition of coordinating groups alpha to the oxaziridine ring as oxaziridines 3b and 3c. In these instances it is proposed that the reaction proceeds through a closed transition state where the metal oxyanion is stabilized by chelation
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
from the sulfate and coordinating groups on the camphor skeleton.
 α-Hydroxylation with oxaziridines has been widely implemented in total synthesis. It is a key step in both the Holton Taxol total synthesis and the Wender Taxol total synthesis.
α-Hydroxylation with oxaziridines has been widely implemented in total synthesis. It is a key step in both the Holton Taxol total synthesis and the Wender Taxol total synthesis.
Epoxidation of alkenes
In academic research, oxaziridines epoxidize many unfunctionalized alkenes stereospecifically. The reaction can be performed catalytically in the oxaziridine whilst still stereospecific, as in the followingoxone
Potassium peroxymonosulfate is widely used as an oxidizing agent, for example, in pools and spas (usually referred to as monopersulfate or "MPS"). It is the potassium salt of peroxymonosulfuric acid. Potassium peroxymonosulfate per se is rarely e ...
-powered epoxidation:
 Further investigation into these reactions may be required before levels of enantiometic excess become practical for large scale synthesis.
Oxaziridines can also form highly acid-sensitive epoxides, as in the following conclusion to a (−)-chaetominine synthesis:
Further investigation into these reactions may be required before levels of enantiometic excess become practical for large scale synthesis.
Oxaziridines can also form highly acid-sensitive epoxides, as in the following conclusion to a (−)-chaetominine synthesis:

Hydroxylation of unactivated hydrocarbons
Perfluorinated oxaziridines hydroxylate unactivated hydrocarbons with remarkable regio- and diastereospecificity. Perfluorinated oxaziridines show high selectivity towardtertiary
Tertiary (from Latin, meaning 'third' or 'of the third degree/order..') may refer to:
* Tertiary period, an obsolete geologic period spanning from 66 to 2.6 million years ago
* Tertiary (chemistry), a term describing bonding patterns in organic ch ...
hydrogens. Hydroxylation of primary carbons and dihydroxylation of a compound with two oxidizable sites have never been observed. Retention of stereochemistry is very high, often 95 to 98%, and often further enhanced by the addition of a fluoride salt.

Nitrogen transfer
Oxaziridines with unsubstituted or acylated nitrogens are capable of nitrogen atom transfer, although this reactivity has received considerably less attention.Amination of ''N''-nucleophiles
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly hazardous unless handled in solution as, for example, hydraz ...
s may be derived from the amination of secondary or tertiary amines, hydroxylamine and thiohydroxamines may be formed from their corresponding alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
s and thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl grou ...
s, sulfimides may be formed from thioether
In organic chemistry, a sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, Volatile organic compound, volatile sulfides have ...
s and α-aminoketones may be formed by attack of corresponding enolates.

''N''-acylamidation
The transfer of acylated amines is more difficult than that of unsubstituted amines. Unlike amine transfer by oxaziridines, there are no alternative methods that directly transfer acylated amines. Acylamine transfer has primarily been performed using amines and hydrazines as nucleophiles. Very few transfers of acylated nitrogens to carbon nucleophiles have been successfully performed, although some do exist in the literature.
Rearrangements
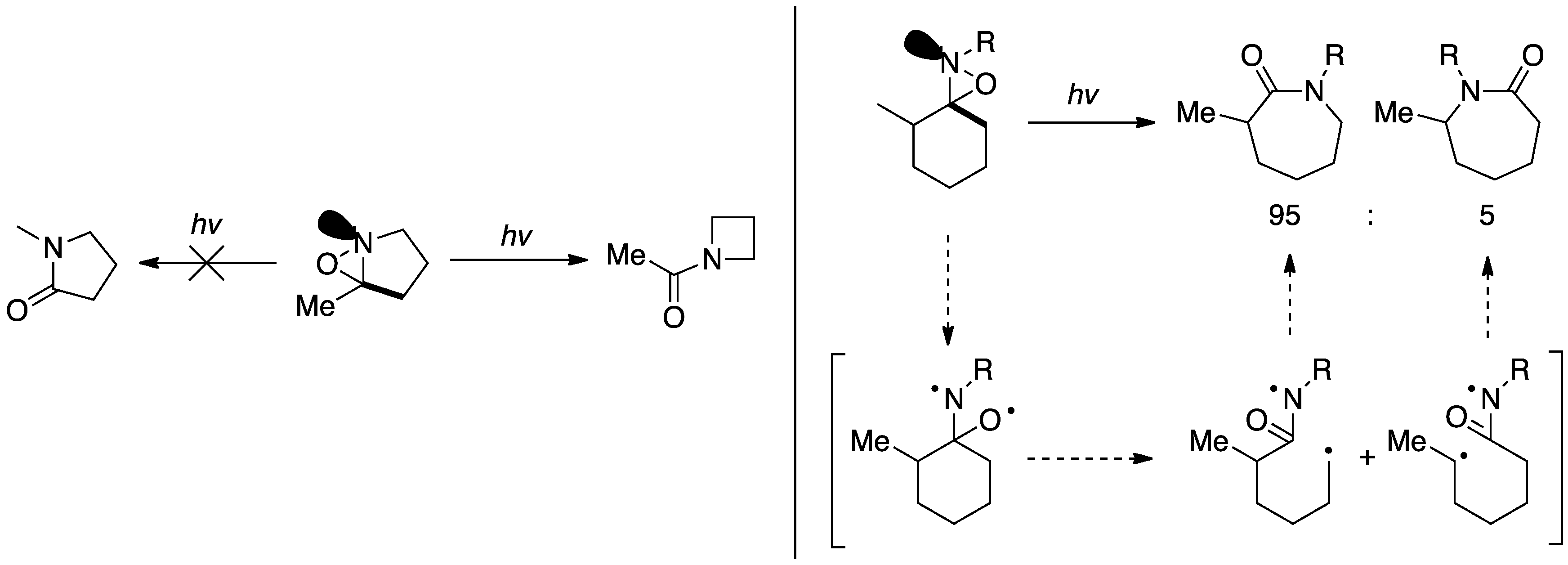
Oxaziridines have been found to undergo rearrangement reactions via a radical mechanism when irradiated with UV light or in the presence of a single electron transfer reagent such as CuI. spirocylic oxaziridines undergo ring expansions to the correspondinglactam
A lactam is a Cyclic compound, cyclic amide, formally derived from an amino alkanoic acid through cyclization reactions. The term is a portmanteau of the words ''lactone'' + ''amide''.
Nomenclature
Greek_alphabet#Letters, Greek prefixes in alpha ...
. The migrating substituent is determined by a stereoelectronic effect
In chemistry, primarily Organic chemistry, organic and computational chemistry, a stereoelectronic effectAlabugin, I. V. Stereoelectronic Effects: the Bridge between Structure and Reactivity. John Wiley & Sons Ltd, Chichester, UK, 2016. http://eu ...
where the group trans to the lone pair on the nitrogen will always be the predominant migration product. In light of this effect, it is possible to take advantage of the chiral nitrogen due to high inversion barrier to direct the rearrangement. This phenomenon is demonstrated by observed selectivities in the rearrangements below. In the rearrangement on the left the thermodynamically unfavorable product is observed exclusively, while in the reaction on the right the product derived from the less stable radical intermediate is favored.
 Aubé takes advantage of this rearrangement as the key step in his synthesis of (+)-
Aubé takes advantage of this rearrangement as the key step in his synthesis of (+)-yohimbine
Yohimbine, also known as quebrachine, is an indole alkaloid derived from the bark of the African tree '' Pausinystalia johimbe'' (yohimbe); also from the bark of the unrelated South American tree '' Aspidosperma quebracho-blanco''. Yohimbine is ...
, a natural medicine classified by the NIH
The National Institutes of Health (NIH) is the primary agency of the United States government responsible for biomedical and public health research. It was founded in 1887 and is part of the United States Department of Health and Human Service ...
as possibly effective in the treatment of erectile dysfunction
Erectile dysfunction (ED), also referred to as impotence, is a form of sexual dysfunction in males characterized by the persistent or recurring inability to achieve or maintain a Human penis, penile erection with sufficient rigidity and durat ...
and the sexual problems caused by selective serotonin reuptake inhibitor
Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions.
SSRIs primarily work by blo ...
s.
 It is also notable that oxaziridines will thermally rearrange to nitrones. Cis-trans selectivity of the resulting nitrone is poor, however, yields are good to excellent. It is thought that some oxaziridines racemize over time through a nitrone intermediate.
It is also notable that oxaziridines will thermally rearrange to nitrones. Cis-trans selectivity of the resulting nitrone is poor, however, yields are good to excellent. It is thought that some oxaziridines racemize over time through a nitrone intermediate.

Cycloadditions with heterocumulenes
Oxaziridines undergocycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
reactions with heterocumulene
A cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumu ...
s to afford a number of unique five membered heterocycles, as shown in the figure below. This reactivity is due to the strained three membered ring and weak N-O bond.

Synthesis
N-H, N-alkyl, N-aryloxaziridines
The two main syntheses of N-H, N-alkyl, and N-aryloxaziridines areimine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bon ...
oxidation with peracids (A) and carbonyl
In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ...
amination (B).
 Oxidation of chiral imines and oxidation of imines with chiral peracids may yield enantiopure oxaziridines.
Oxidation of chiral imines and oxidation of imines with chiral peracids may yield enantiopure oxaziridines.
''N''-sulfonyloxaziridines
Many N-sulfonyloxaziridines are used today, each with slightly different properties and reactivity. These reagents are summarized in the table below. While originally synthesized with mCPBA and thephase transfer catalyst
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of catalysis and can act through homog ...
benzyltrimethylammonium chloride, an improved synthesis using oxone
Potassium peroxymonosulfate is widely used as an oxidizing agent, for example, in pools and spas (usually referred to as monopersulfate or "MPS"). It is the potassium salt of peroxymonosulfuric acid. Potassium peroxymonosulfate per se is rarely e ...
as the oxidant is now most prevalent.
Perfluorinated oxaziridines
With highly electron withdrawing perfluoroalkyl substituents, oxaziridines react more similarly todioxirane
In chemistry, dioxirane (systematically named dioxacyclopropane, also known as methylene peroxide or peroxymethane) is an organic compound with formula . The molecule consists of a ring with one methylene and two oxygen atoms. It is of interes ...
s . Notably, perfluoroalkyloxaziridines hydroxylate certain C-H bonds with high selectivity. Perfluorinated oxaziridines may be synthesized by subjecting a perfluorinated imine to perfluoromethyl fluorocarbonyl peroxide and a metal fluoride to act as an HF scavenger.

References
{{oxygen compounds Functional groups Nitrogen heterocycles Oxygen heterocycles Reagents for organic chemistry Three-membered rings