|
Cyclopropenium
The cyclopropenium ion is the cation with the formula . It has attracted attention as the smallest example of an aromatic cation. Its salts have been isolated, and many derivatives have been characterized by X-ray crystallography. The cation and some simple derivatives have been identified in the atmosphere of the Saturnian moon Titan. Bonding With two π electrons, the cyclopropenium cation class obeys Hückel’s rules of aromaticity for electrons since, in this case, ''n'' = 0. Consistent with this prediction, the C3H3 core is planar and the C–C bonds are equivalent. In the case of the cation in 3(SiMe3)3sup>+, the ring C–C distances range from 1.374(2) to 1.392(2) Å. Syntheses Salts of many cyclopropenyl cations have been characterized. Their stability varies according to the steric and inductive effects of the substituents. Salts of triphenylcyclopropenium were first reported by Ronald Breslow in 1957. The salt was prepared in two steps starting with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a Transition metal alkyne complex, ligand in organometallic chemistry. Preparation and structure In one preparation for this compound, benzil is condensed with hydrazine to give the bis(hydrazone), which is oxidized with mercury(II) oxide. Alternatively stilbene is brominated, and the resulting dibromodiphenylethane is subjected to dehydrohalogenation. Yet another method involves the coupling of iodobenzene and the copper salt of phenylacetylene in the Castro-Stephens coupling. The related Sonogashira coupling involves the coupling of iodobenzene and phenylacetylene. Diphenylacetylene is a planar molecule. The central C≡C distance is 119.8 picometers. Derivatives 1,2,3,4-Tetraphenylbutadiene is prepared by reductive coupling of diphenylacetylene using lithium metal followed by h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrachlorocyclopropene
Tetrachlorocyclopropene is a chemical compound with the formula C3Cl4. A colorless liquid, the compound is a reagent used to prepare acetylene derivatives and in organic synthesis. It is prepared by addition of dichlorocarbene to trichloroethylene. It can react with water and alcohols rapidly.Pentachlorocyclopropane Stephen W. Tobey and Robert West. The University of Wisconsin (1965) The compound is used to prepare arylpropiolic acids: :C3Cl4 + ArH + 2 H2O → ArC2CO2H + 4 HCl Under some circumstances, diarylation occurs, giving diarylcyclopropenones, which decarbonylate to give diarylacetylenes. These reactions are thought to proceed via the intermediacy of trichlorocyclopropenium The cyclopropenium ion is the cation with the formula . It has attracted attention as the smallest example of an aromatic cation. Its salts have been isolated, and many derivatives have been characterized by X-ray crystallography. The cation and so ... electrophile (C3Cl3+). References Or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
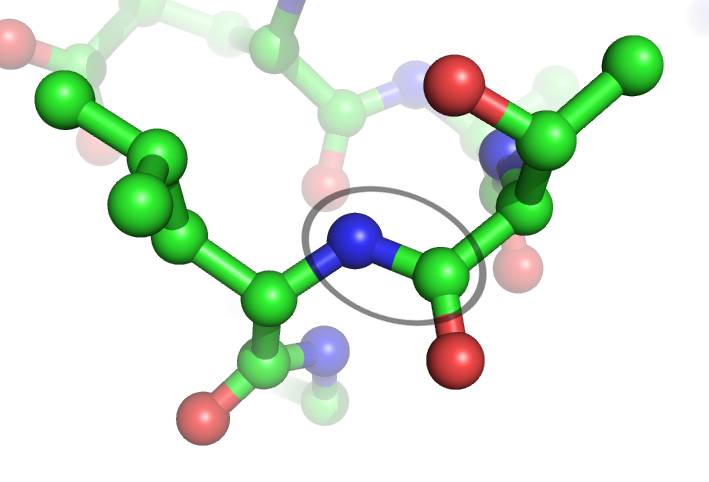
Peptide Bond
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one) of one alpha-amino acid and N2 (nitrogen number two) of another, along a peptide or protein chain. It can also be called a eupeptide bond to distinguish it from an isopeptide bond, which is another type of amide bond between two amino acids. Synthesis When two amino acids form a '' dipeptide'' through a ''peptide bond'', it is a type of condensation reaction. In this kind of condensation, two amino acids approach each other, with the non-side chain (C1) carboxylic acid moiety of one coming near the non-side chain (N2) amino moiety of the other. One loses a hydrogen and oxygen from its carboxyl group (COOH) and the other loses a hydrogen from its amino group (NH2). This reaction produces a molecule of water (H2O) and two amino acids joined by a peptide bond (−CO−NH−). The two joined amino acids are called a dipeptide. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopropenone
Cyclopropenone is an organic compound with molecular formula consisting of a cyclopropene carbon framework with a ketone functional group. It is a colorless, volatile liquid that boils near room temperature. Neat cyclopropenone polymerizes upon standing at room temperature, and chemical vendors typically supply it as an acetal. The chemical properties of the compound are dominated by the strong polarization of the carbonyl In organic chemistry, a carbonyl group is a functional group with the formula , composed of a carbon atom double bond, double-bonded to an oxygen atom, and it is divalent at the C atom. It is common to several classes of organic compounds (such a ... group, which gives a partial positive charge with aromatic stabilization on the ring and a partial negative charge on oxygen. It is an aromatic compound. See also * Diphenylcyclopropenone * Deltic acid * Tropone References {{Molecules detected in outer space Enones Simple aromatic rings Cyclopropenes No ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example of an acyl chloride is acetyl chloride, . Acyl chlorides are the most important subset of acyl halides. Nomenclature Where the acyl chloride Moiety (chemistry), moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting ''-yl chloride'' for ''-ic acid''. Thus: : : :Butyric acid, butyr''ic acid'' (C3H7COOH) → Butyryl chloride, butyr''yl chloride'' (C3H7COCl) (Idiosyncratically, for some trivial names, ''-oyl chloride'' substitutes ''-ic acid''. For example, pival''ic acid'' becomes pival''oyl chloride'' and acryl''ic acid'' becomes acryl''oyl chloride.'' The names pivalyl chloride and acrylyl chloride are less commonly used, although they are arguably more logical.) When other fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl group (e.g., alkyl, alkenyl, aryl), or hydrogen, or other groups. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They often have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid () is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |