|
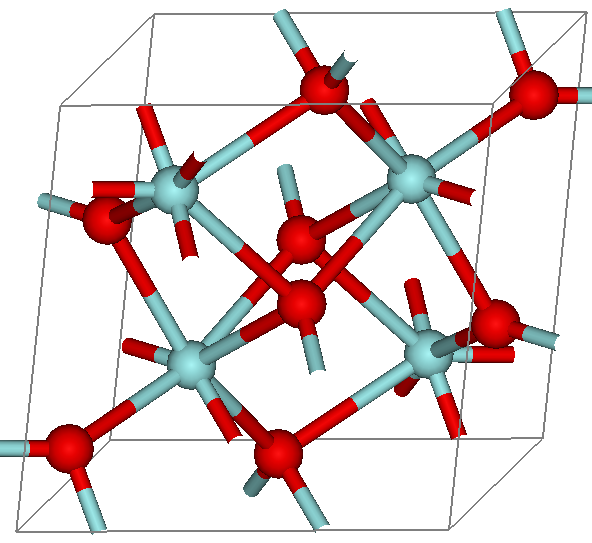
Cubic Zirconia
Cubic zirconia (CZ) is the cubic crystalline form of zirconium dioxide (ZrO2). The synthesized material is hard and usually colorless, but may be made in a variety of different colors. It should not be confused with zircon, which is a zirconium silicate (ZrSiO4). It is sometimes erroneously called ''cubic zirconium''. Because of its low cost, durability, and close visual likeness to diamond, synthetic cubic zirconia has remained the most gemologically and economically important competitor for diamonds since commercial production began in 1976. Its main competitor as a synthetic gemstone is a more recently cultivated material, synthetic moissanite. Technical aspects Cubic zirconia is crystallographically isometric, an important attribute of a would-be diamond simulant. During synthesis zirconium oxide naturally forms monoclinic crystals, which are stable under normal atmospheric conditions. A stabilizer is required for cubic crystals (taking on the fluorite structure) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxide Minerals
The oxide mineral class includes those minerals in which the oxide anion (O2−) is bonded to one or more metal alloys. The hydroxide-bearing minerals are typically included in the oxide class. Minerals with complex anion groups such as the Silicate mineral, silicates, Sulfate mineral, sulfates, carbonate mineral, carbonates and Phosphate mineral, phosphates are classed separately. Simple oxides *XO form **Periclase group ***Periclase ***Manganosite **Zincite group ***Zincite ***Bromellite ***Tenorite ***Litharge * form **Cuprite **Ice * form **Hematite group ***Corundum ***Hematite ***Ilmenite * form **Rutile group ***Rutile ***Pyrolusite ***Cassiterite **Baddeleyite **Uraninite **Thorianite * form **Spinel group ***Spinel ***Gahnite ***Magnetite ***Franklinite ***Chromite **Chrysoberyl **Columbite *Hydroxide subgroup: **Brucite **Manganite **Romanèchite **Goethite group: ***Diaspore ***Goethite Nickel–Strunz class 4: oxides Internationa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the ratio of a substance's mass to its volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' (or ''d'') can also be used: \rho = \frac, where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate this quantity is more specifically called specific weight. For a pure substance, the density is equal to its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium is the densest known element at standard conditions for temperature and pressure. To simplify comparisons of density across different systems of units, it is sometimes replaced by the dimensionless quantity "relative den ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, and brick. The earliest ceramics made by humans were fired clay bricks used for building house walls and other structures. Other pottery objects such as pots, vessels, vases and figurines were made from clay, either by itself or mixed with other materials like silica, hardened by sintering in fire. Later, ceramics were glazed and fired to create smooth, colored surfaces, decreasing porosity through the use of glassy, amorphous ceramic coatings on top of the crystalline ceramic substrates. Ceramics now include domestic, industrial, and building products, as well as a wide range of materials developed for use in advanced ceramic engineering, such as semiconductors. The word '' ceramic'' comes from the Ancient Greek word (), meaning ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycrystalline
A crystallite is a small or even microscopic crystal which forms, for example, during the cooling of many materials. Crystallites are also referred to as grains. Bacillite is a type of crystallite. It is rodlike with parallel longulites. Structure The orientation of crystallites can be random with no preferred direction, called random texture, or directed, possibly due to growth and processing conditions. While the structure of a single crystal is highly ordered and its lattice is continuous and unbroken, amorphous materials, such as glass and many polymers, are non-crystalline and do not display any structures, as their constituents are not arranged in an ordered manner. Polycrystalline structures and paracrystalline phases are in between these two extremes. Polycrystalline materials, or polycrystals, are solids that are composed of many crystallites of varying size and orientation. Most materials are polycrystalline, made of a large number crystallites held together by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baddeleyite
Baddeleyite is a rare zirconium oxide mineral (ZrO2 or zirconia), occurring in a variety of monoclinic prismatic crystal forms. It is transparent to translucent, has high Index of refraction, indices of refraction, and ranges from colorless to yellow, green, and dark brown. See etymology Baddeleyite#Origin of the name, below. Baddeleyite is a refractory mineral, with a melting point of 2700 °C. Hafnium is a substituting ''impurity'' and may be present in quantities ranging from 0.1 to several percent. It can be found in igneous rocks containing potassium feldspar and plagioclase. Baddeleyite is commonly not found with zircon (ZrSiO4), because it forms in silica-undersaturated rocks, such as mafic rocks. This is because, when silica is free in the system (silica-saturated/oversaturated), zircon is the dominating phase, not baddeleyite. It belongs to the Monoclinic crystal system, monoclinic-prismatic class, of the P21/c crystal system. It has been used for geochronology. Geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption Spectrum
Absorption spectroscopy is spectroscopy that involves techniques that measure the absorption of electromagnetic radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum. Absorption spectroscopy is employed as an analytical chemistry tool to determine the presence of a particular substance in a sample and, in many cases, to quantify the amount of the substance present. Infrared and ultraviolet–visible spectroscopy are particularly common in analytical applications. Absorption spectroscopy is also employed in studies of molecular and atomic physics, astronomical spectroscopy and remote sensing. There is a wide range of experimental approaches for measuring absorption spectra. The most common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare Earth Element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of 17 nearly indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. The term "rare-earth" is a misnomer because they are not actually scarce, but historically it took a long time to isolate these elements. They are relatively plentiful in the entire Earth's crust ( cerium being the 25th-most-abundant element at 68 parts per million, more abundant than copper), but in practice they are spread thinly as trace impurities, so to obtain rare earths at usable purity requires processing enormous amounts of raw ore at great expense; thus the name "rare" earths. Scandium and yttrium a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission, phosphorescence. Phosphorescent materials continue to emit light for some time after the radiation stops. This difference in duration is a result of quantum spin effects. Fluorescence occurs when a photon from incoming radiation is absorbed by a molecule, exciting it to a higher energy level, followed by the emission of light as the molecule returns to a lower energy state. The emitted light may have a longer wavelength and, therefore, a lower photon energy than the absorbed radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brittleness
A material is brittle if, when subjected to stress (physics), stress, it fractures with little elastic deformation and without significant plastic deformation. Brittle materials absorb relatively little energy prior to fracture, even those of high strength of materials, strength. Breaking is often accompanied by a sharp snapping sound. When used in materials science, it is generally applied to materials that fail when there is little or no plasticity (physics), plastic deformation before failure. One proof is to match the broken halves, which should fit exactly since no plastic deformation has occurred. Brittleness in different materials Polymers Mechanical characteristics of polymers can be sensitive to temperature changes near room temperatures. For example, poly(methyl methacrylate) is extremely brittle at temperature 4˚C, but experiences increased ductility with increased temperature. Amorphous polymers are polymers that can behave differently at different temperatures. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conchoidal Fracture
A conchoidal fracture is a break or fracture of a brittle material that does not follow any natural planes of separation. Mindat.org defines ''conchoidal fracture'' as follows: "a fracture with smooth, curved surfaces, typically slightly concave, showing concentric undulations resembling the lines of growth of a shell".Conchoidal fracture at Mindat.org Materials that break in this way include , chert, , [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cleavage (crystal)
Cleavage, in mineralogy and materials science, is the tendency of crystalline materials to split along definite crystallographic structural planes. These planes of relative weakness are a result of the regular locations of atoms and ions in the crystal, which create smooth repeating surfaces that are visible both in the microscope and to the naked eye. If bonds in certain directions are weaker than others, the crystal will tend to split along the weakly bonded planes. These flat breaks are termed "cleavage".Hurlbut, Cornelius S.; Klein, Cornelis, 1985, '' Manual of Mineralogy'', 20th ed., Wiley, The classic example of cleavage is mica, which cleaves in a single direction along the basal pinacoid, making the layers seem like pages in a book. In fact, mineralogists often refer to "books of mica". Diamond and graphite provide examples of cleavage. Each is composed solely of a single element, carbon. In diamond, each carbon atom is bonded to four others in a tetrahedral patter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
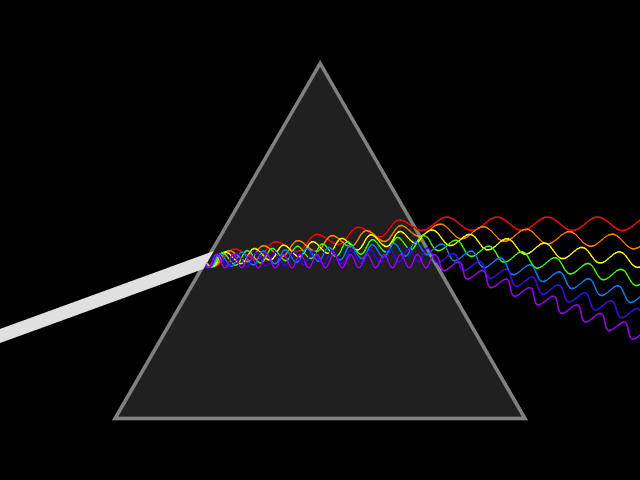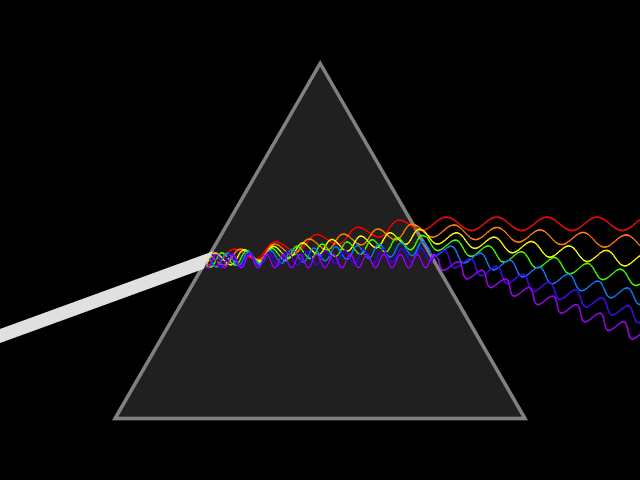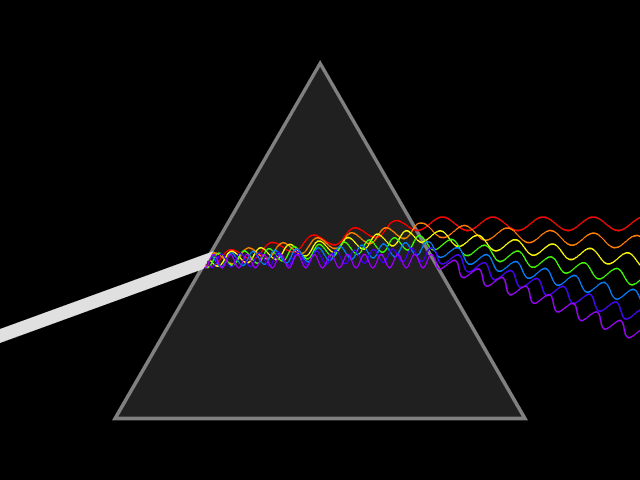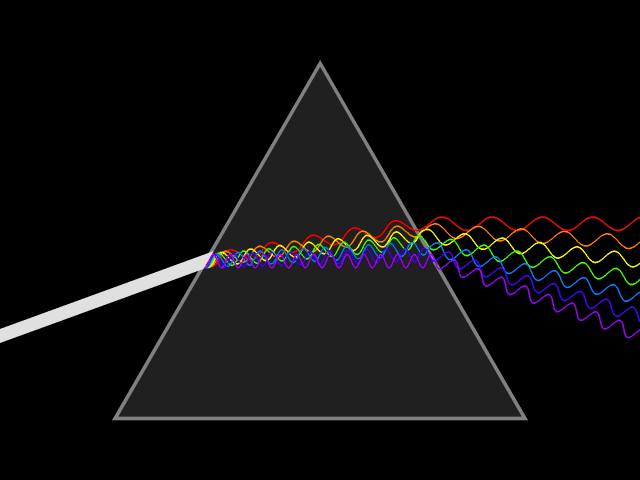
Dispersion (optics)
Dispersion is the phenomenon in which the phase velocity of a wave depends on its frequency. Sometimes the term chromatic dispersion is used to refer to optics specifically, as opposed to wave propagation in general. A medium having this common property may be termed a dispersive medium. Although the term is used in the field of optics to describe light and other electromagnetic waves, dispersion in the same sense can apply to any sort of wave motion such as acoustic dispersion in the case of sound and seismic waves, and in gravity waves (ocean waves). Within optics, dispersion is a property of telecommunication signals along transmission lines (such as microwaves in coaxial cable) or the Pulse (signal processing), pulses of light in optical fiber. In optics, one important and familiar consequence of dispersion is the change in the angle of refraction of different colors of light, as seen in the spectrum produced by a dispersive Prism (optics), prism and in chromatic aberration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |