|
Costin Nenițescu
Costin D. Nenițescu (in some places ''Nenitzescu'' (; 15 July 1902 – 28 July 1970)) was a prominent Romanian chemist, and a professor at the Politehnica University of Bucharest. He was a titular list of members of the Romanian Academy, member of the Romanian Academy, a corresponding member of the German Academy of Sciences at Berlin, German Academy of Sciences in Berlin, and a member of the German National Academy of Sciences Leopoldina, Leopoldina Academy of Natural Scientists in Halle, Saxony-Anhalt, Halle-Saale. Early life He was born in Bucharest, the son of (lawyer and politician, director of the National Bank of Romania and Minister of Industry and Commerce) and Elena Nenițescu, and the nephew of the writer Ioan S. Nenițescu. After completing in 1920 his secondary studies at Gheorghe Lazăr National College (Bucharest), Gheorghe Lazăr High School, Nenițescu continued his studies at the ETH Zurich, Polytechnic Institute in Zurich and Ludwig Maximilian University of M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bucharest
Bucharest ( , ; ) is the capital and largest city of Romania. The metropolis stands on the River Dâmbovița (river), Dâmbovița in south-eastern Romania. Its population is officially estimated at 1.76 million residents within a greater Bucharest metropolitan area, metropolitan area of 2.3 million residents, which makes Bucharest the List of cities in the European Union by population within city limits, 8th most-populous city in the European Union. The city area measures and comprises 6 districts (''Sectors of Bucharest, Sectoare''), while the metropolitan area covers . Bucharest is a major cultural, political and economic hub, the country's seat of government, and the capital of the Muntenia region. Bucharest was first mentioned in documents in 1459. The city became the capital in 1862 and is the centre of Romanian media, culture, and art. Its architecture is a mix of historical (mostly History of architecture#Revivalism and Eclecticism, Eclectic, but also Neoclassical arc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
German National Academy Of Sciences Leopoldina
The German National Academy of Sciences Leopoldina (), in short Leopoldina, is the national academy of Germany, and is located in Halle (Saale). Founded on 1 January 1652, based on academic models in Italy, it was originally named the ''Academia Naturae Curiosorum'' until 1687 when Emperor Leopold I raised it to an academy and named it after himself. It was since known under the German name ''Deutsche Akademie der Naturforscher Leopoldina'' until 2007, when it was declared to be Germany's National Academy of Sciences. It is the oldest continuously operating academy of natural sciences worldwide. History ' The Leopoldina was founded in the imperial city of Schweinfurt on 1 January 1652 under the Latin name sometimes translated into English as "Academy of the Curious as to Nature." It was founded by four local physicians – Johann Laurentius Bausch, the first president of the society, Johann Michael Fehr, Georg Balthasar Metzger, and Georg Balthasar Wohlfarth; and was the on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrylium Salt
Pyrylium is a cation (positive ion) with formula , consisting of a six-membered ring of five carbon atoms, each with one hydrogen atom, and one positively charged oxygen atom. The bonds in the ring are conjugated as in benzene, giving it an aromatic character. In particular, because of the positive charge, the oxygen atom is trivalent. Pyrilium is a mono- cyclic and heterocyclic compound, one of the oxonium ions. Synthesis Pyrylium salts are easily produced from simple starting materials through a condensation reaction. Pyrylium salts with aromatic substituents, such 2,4,6-triphenylpyrylium tetrafluoroborate, can be obtained from two moles of acetophenone, one mole of benzaldehyde, and excess tetrafluoroboric acid. For pyrylium salts with alkyl substituents, such as 2,4,6-trimethylpyrylium salts, the best method uses the Balaban- Nenitzescu-Praill synthesis from tertiary butanol and acetic anhydride in the presence of tetrafluoroboric, perchloric, or trifluoromethanesulfon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromic Oxychloride
Chromyl chloride is an inorganic compound with the formula CrO2Cl2. It is a reddish brown compound that is a volatile liquid at room temperature, which is unusual for transition metal compounds. It is the dichloride of chromic acid. Preparation Chromyl chloride can be prepared by the reaction of potassium chromate or potassium dichromate with hydrogen chloride in the presence of concentrated sulfuric acid, followed by distillation. :K2Cr2O7 + 6 HCl → 2 CrO2Cl2 + 2 KCl + 3 H2O The sulfuric acid serves as a dehydration agent. It can also be prepared directly by exposing chromium trioxide to anhydrous hydrogen chloride gas. :CrO3 + 2 HCl ⇌ CrO2Cl2 + H2O Usage Test for the presence of chlorides The chromyl chloride test involves heating a sample suspected to contain chlorides with potassium dichromate and concentrated sulfuric acid. If a chloride is present then chromyl chloride forms which is indicated by the evolution of red smoke. No similar compound is formed in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromic Acid
Chromic acid is a chemical compound with the chemical formula . It is also a jargon for a solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide. The term "chromic acid" is usually used for a mixture made by adding concentrated sulfuric acid to a dichromate, which may contain a variety of compounds, including solid chromium trioxide. This kind of chromic acid may be used as a cleaning mixture for glass. Chromic acid may also refer to the molecular species, of which the trioxide is the anhydride. Chromic acid features chromium in an oxidation state of +6 (and a valence of VI or 6). It is a strong and corrosive oxidizing agent and a moderate carcinogen. Molecular chromic acid Molecular chromic acid, , in principle, resembles sulfuric acid, . It would ionize accordingly: : The p''K''a for the equilibrium is not well characterized. Reported values vary between about −0.8 to 1.6. The structur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Hydrocarbons
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's rule. Aromatic compounds have the following general properties: * Typically unreactive * Often non polar and hydrophobic * High carbon-hydrogen ratio * Burn with a strong sooty yellow flame, due to high C:H ratio * Undergo electrophilic substitution reactions and nucleophilic aromatic substitutions Arenes are typically split into two categories - benzoids, that contain a benzene derivative and follow the benzene ring model, and non-benzoids that contain other aromatic cyclic derivatives. Aromatic compounds are commonly used in organic synthesis and are involved in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
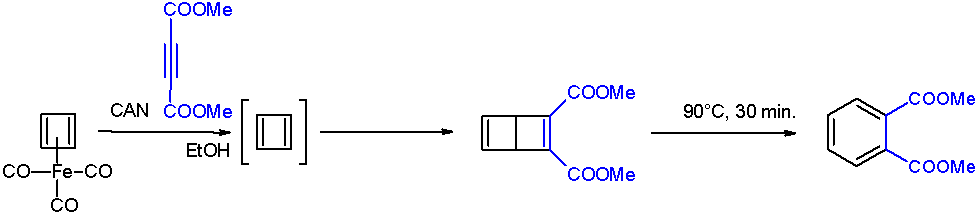
Cyclobutadiene
Cyclobutadiene is an organic compound with the formula . It is very reactive owing to its tendency to dimerize. Although the parent compound has not been isolated, some substituted derivatives are robust and a single molecule of cyclobutadiene is quite stable. Since the compound degrades by a bimolecular process, the species can be observed by matrix isolation techniques at temperatures below 35 K. It is thought to adopt a rectangular structure. Structure and reactivity The compound is the prototypical antiaromatic hydrocarbon with 4 pi electrons (or π electrons). It is the smallest 'n''annulene ( annulene). Its rectangular structure is the result of a pseudo- (or second order) Jahn–Teller effect, which distorts the molecule and lowers its symmetry, converting the triplet to a singlet ground state. The electronic states of cyclobutadiene have been explored with a variety of computational methods. The rectangular structure is consistent with the existence of two different ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthenic Acid
Naphthenic acids (NAs) are mixtures of several cyclopentyl and cyclohexyl carboxylic acids with molecular weights of 120 to well over 700 atomic mass units. The main fractions are carboxylic acids with a carbon backbone of 9 to 20 carbons. McKee et al. claim that "naphthenic acids (NAs) are primarily cycloaliphatic carboxylic acids with 10 to 16 carbons", although acids containing up to 50 carbons have been identified in heavy petroleum.Qian, K. and W.K. Robbins (2001). Resolution and identification of elemental compositions for more than 3000 crude acids in heavy petroleum by negative-ion microelectrospray high-field Fourier Transform ion cyclotron resonance mass spectrometry. ''Energy & Fuels.'' 15:1505-1511. Nomenclature Naphthenic acid can refer to derivatives and isomers of naphthalene carboxylic acids. In the petrochemical industry, NA's refer to alkyl carboxylic acids found in petroleum. The term naphthenic acid has roots in the somewhat archaic term "naphthene" (cycloalip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aliphatic Compound
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated (in which all the C-C bonds are single, requiring the structure to be completed, or 'saturated', by hydrogen) like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic. Structure Aliphatics compounds can be saturated, joined by single bonds ( alkanes), or unsaturated, with double bonds ( alkenes) or triple bonds ( alkynes). If other elements ( heteroatoms) are bound to the carbon chain, the most common being oxygen, nitrogen, sulfur, and chlorine, it is no longer a hydrocarbon, and therefore no longer an aliphatic compound. However, such compounds may still be referred to as al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Munich
Munich is the capital and most populous city of Bavaria, Germany. As of 30 November 2024, its population was 1,604,384, making it the third-largest city in Germany after Berlin and Hamburg. Munich is the largest city in Germany that is not a state of its own. It ranks as the 11th-largest city in the European Union. The metropolitan area has around 3 million inhabitants, and the broader Munich Metropolitan Region is home to about 6.2 million people. It is the List of EU metropolitan regions by GDP#2021 ranking of top four German metropolitan regions, third largest metropolitan region by GDP in the European Union. Munich is located on the river Isar north of the Alps. It is the seat of the Upper Bavaria, Upper Bavarian administrative region. With 4,500 people per km2, Munich is Germany's most densely populated municipality. It is also the second-largest city in the Bavarian language, Bavarian dialect area after Vienna. The first record of Munich dates to 1158. The city ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zurich
Zurich (; ) is the list of cities in Switzerland, largest city in Switzerland and the capital of the canton of Zurich. It is in north-central Switzerland, at the northwestern tip of Lake Zurich. , the municipality had 448,664 inhabitants. The Urban agglomeration, urban area was home to 1.45 million people (2020), while the Zurich Metropolitan Area, Zurich metropolitan area had a total population of 2.1 million (2020). Zurich is a hub for railways, roads, and air traffic. Both Zurich Airport and Zürich Hauptbahnhof, Zurich's main railway station are the largest and busiest in the country. Permanently settled for over 2,000 years, Zurich was founded by the Roman Empire, Romans, who called it '. However, early settlements have been found dating back more than 6,400 years (although this only indicates human presence in the area and not the presence of a town that early). During the Middle Ages, Zurich gained the independent and privileged status of imperial immediacy and, in 1519 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |