|
Copenhagen School (quantum Physics)
The Copenhagen interpretation is a collection of views about the meaning of quantum mechanics, stemming from the work of Niels Bohr, Werner Heisenberg, Max Born, and others. While "Copenhagen" refers to the Danish city, the use as an "interpretation" was apparently coined by Heisenberg during the 1950s to refer to ideas developed in the 1925–1927 period, glossing over his disagreements with Bohr. Consequently, there is no definitive historical statement of what the interpretation entails. Features common across versions of the Copenhagen interpretation include the idea that quantum mechanics is intrinsically Indeterminism, indeterministic, with probabilities calculated using the Born rule, and the principle of Complementarity (physics), complementarity, which states that objects have certain pairs of complementary properties that cannot all be observed or measured simultaneously. Moreover, the act of "observing" or "measuring" an object is irreversible, and no truth can be attr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is the foundation of all quantum physics, which includes quantum chemistry, quantum field theory, quantum technology, and quantum information science. Quantum mechanics can describe many systems that classical physics cannot. Classical physics can describe many aspects of nature at an ordinary (macroscopic and Microscopic scale, (optical) microscopic) scale, but is not sufficient for describing them at very small submicroscopic (atomic and subatomic) scales. Classical mechanics can be derived from quantum mechanics as an approximation that is valid at ordinary scales. Quantum systems have Bound state, bound states that are Quantization (physics), quantized to Discrete mathematics, discrete values of energy, momentum, angular momentum, and ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Albert Einstein
Albert Einstein (14 March 187918 April 1955) was a German-born theoretical physicist who is best known for developing the theory of relativity. Einstein also made important contributions to quantum mechanics. His mass–energy equivalence formula , which arises from special relativity, has been called "the world's most famous equation". He received the 1921 Nobel Prize in Physics for . Born in the German Empire, Einstein moved to Switzerland in 1895, forsaking his German citizenship (as a subject of the Kingdom of Württemberg) the following year. In 1897, at the age of seventeen, he enrolled in the mathematics and physics teaching diploma program at the Swiss ETH Zurich, federal polytechnic school in Zurich, graduating in 1900. He acquired Swiss citizenship a year later, which he kept for the rest of his life, and afterwards secured a permanent position at the Swiss Patent Office in Bern. In 1905, he submitted a successful PhD dissertation to the University of Zurich. In 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matrix (mathematics)
In mathematics, a matrix (: matrices) is a rectangle, rectangular array or table of numbers, symbol (formal), symbols, or expression (mathematics), expressions, with elements or entries arranged in rows and columns, which is used to represent a mathematical object or property of such an object. For example, \begin1 & 9 & -13 \\20 & 5 & -6 \end is a matrix with two rows and three columns. This is often referred to as a "two-by-three matrix", a " matrix", or a matrix of dimension . Matrices are commonly used in linear algebra, where they represent linear maps. In geometry, matrices are widely used for specifying and representing geometric transformations (for example rotation (mathematics), rotations) and coordinate changes. In numerical analysis, many computational problems are solved by reducing them to a matrix computation, and this often involves computing with matrices of huge dimensions. Matrices are used in most areas of mathematics and scientific fields, either directly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Über Quantentheoretische Umdeutung Kinematischer Und Mechanischer Beziehungen
In the history of physics, "On the quantum-theoretical reinterpretation of kinematical and mechanical relationships" (), also known as the ''Umdeutung'' (reinterpretation) paper, was a breakthrough article in quantum mechanics written by Werner Heisenberg, which appeared in ''Zeitschrift für Physik'' in September 1925. In the article, Heisenberg tried to explain the energy levels of a one-dimensional anharmonic oscillator, avoiding the concrete but unobservable representations of Electron configuration, electron orbits by using observable parameters such as transition probabilities for quantum jumps, which necessitated using two indexes corresponding to the initial and final states. Mathematically, Heisenberg showed the need of non-commutative operators. This insight would later become the basis for Heisenberg's uncertainty principle. This article was followed by the paper by Max Born and Pascual Jordan of the same year, and by the 'three-man paper' () by Born, Heisenberg and J ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helium Atom
A helium atom is an atom of the chemical element helium. Helium is composed of two electrons bound by the electromagnetic force to a nucleus containing two protons along with two neutrons, depending on the isotope, held together by the strong force. Unlike for hydrogen, a closed-form solution to the Schrödinger equation for the helium atom has not been found. However, various approximations, such as the Hartree–Fock method, can be used to estimate the ground state energy and wavefunction of the atom. Historically, the first attempt to obtain the helium spectrum from quantum mechanics was done by Albrecht Unsöld in 1927. Egil Hylleraas obtained an accurate approximation in 1929. Its success was considered to be one of the earliest signs of validity of Schrödinger's wave mechanics. Introduction The quantum mechanical description of the helium atom is of special interest, because it is the simplest multi-electron system and can be used to understand the concept of q ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Special Relativity
In physics, the special theory of relativity, or special relativity for short, is a scientific theory of the relationship between Spacetime, space and time. In Albert Einstein's 1905 paper, Annus Mirabilis papers#Special relativity, "On the Electrodynamics of Moving Bodies", the theory is presented as being based on just Postulates of special relativity, two postulates: # The laws of physics are Invariant (physics), invariant (identical) in all Inertial frame of reference, inertial frames of reference (that is, Frame of reference, frames of reference with no acceleration). This is known as the principle of relativity. # The speed of light in vacuum is the same for all observers, regardless of the motion of light source or observer. This is known as the principle of light constancy, or the principle of light speed invariance. The first postulate was first formulated by Galileo Galilei (see ''Galilean invariance''). Background Special relativity builds upon important physics ide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bohr Model
In atomic physics, the Bohr model or Rutherford–Bohr model was a model of the atom that incorporated some early quantum concepts. Developed from 1911 to 1918 by Niels Bohr and building on Ernest Rutherford's nuclear Rutherford model, model, it supplanted the plum pudding model of J. J. Thomson only to be replaced by the quantum atomic model in the 1920s. It consists of a small, dense nucleus surrounded by orbiting electrons. It is analogy, analogous to the structure of the Solar System, but with attraction provided by Coulomb's law, electrostatic force rather than gravity, and with the electron energies quantized (assuming only discrete values). In the history of atomic physics, it followed, and ultimately replaced, several earlier models, including Joseph Larmor's Solar System model (1897), Jean Perrin's model (1901), the Cubical atom, cubical model (1902), Hantaro Nagaoka's Saturnian model (1904), the plum pudding model (1904), Arthur Haas's quantum model (1910), the Ru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arnold Sommerfeld
Arnold Johannes Wilhelm Sommerfeld (; 5 December 1868 – 26 April 1951) was a German Theoretical physics, theoretical physicist who pioneered developments in Atomic physics, atomic and Quantum mechanics, quantum physics, and also educated and mentored many students for the new era of theoretical physics. He served as doctoral advisor and Postdoctoral researcher, postdoc advisor to seven Nobel Prize winners and supervised at least 30 other famous physicists and chemists. Only J. J. Thomson's record of mentorship offers a comparable list of high-achieving students. He introduced the second quantum number, azimuthal quantum number, and the third quantum number, magnetic quantum number. He also introduced the fine-structure constant and pioneered X-ray wave theory. Early life and education Sommerfeld was born in 1868 to a family with deep ancestral roots in Prussia. His mother Cäcilie Matthias (1839–1902) was the daughter of a Potsdam builder. His father Franz Sommerfeld (1820� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral hydrogen atom contains a single positively charged proton in the nucleus, and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the baryonic mass of the universe. In everyday life on Earth, isolated hydrogen atoms (called "atomic hydrogen") are extremely rare. Instead, a hydrogen atom tends to combine with other atoms in compounds, or with another hydrogen atom to form ordinary (diatomic) hydrogen gas, H2. "Atomic hydrogen" and "hydrogen atom" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms). Atomic spectroscopy shows that there is a discrete infinite set of states in which a hydrogen (or any) atom can exist, contrary to the predictions of classical physics. At ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
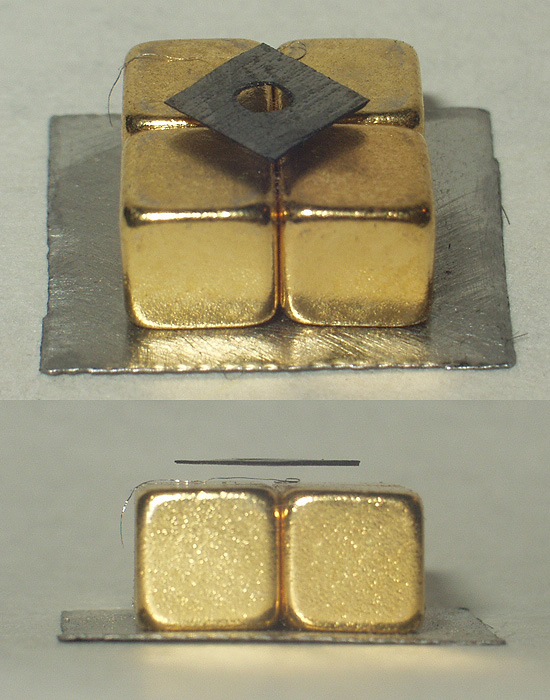
Diamagnetism
Diamagnetism is the property of materials that are repelled by a magnetic field; an applied magnetic field creates an induced magnetic field in them in the opposite direction, causing a repulsive force. In contrast, paramagnetic and ferromagnetic materials are attracted by a magnetic field. Diamagnetism is a quantum mechanical effect that occurs in all materials; when it is the only contribution to the magnetism, the material is called diamagnetic. In paramagnetic and ferromagnetic substances, the weak diamagnetic force is overcome by the attractive force of magnetic dipoles in the material. The Permeability (electromagnetism), magnetic permeability of diamagnetic materials is less than the Vacuum_permeability, permeability of vacuum, ''μ''0. In most materials, diamagnetism is a weak effect which can be detected only by sensitive laboratory instruments, but a superconductivity, superconductor acts as a strong diamagnet because it entirely expels any magnetic field from its inter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hendrika Johanna Van Leeuwen
Hendrika Johanna van Leeuwen (July 3, 1887 – February 26, 1974) was a Dutch physicist known for her early contributions to the theory of magnetism. She studied at Leiden University under the guidance of Hendrik Antoon Lorentz, obtaining her doctorate in 1919. Her thesis explained why magnetism is an essentially quantum mechanical effect, a result now referred to as the Bohr–Van Leeuwen theorem. (Niels Bohr had arrived at the same conclusion a few years earlier.) She continued to investigate magnetic materials at the "Technische Hogeschool Delft" (now called the Delft University of Technology), first as "assistant" between September 1920 and April 1947, and then she was promoted to " lector in de theoretische en toegepaste natuurkunde" (reader in theoretical and applied physics). Hendrika van Leeuwen was the sister-in-law of Gunnar Nordström, known as the "Einstein of Finland", who studied in Leiden with Paul Ehrenfest Paul Ehrenfest (; 18 January 1880 – 25 September ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |