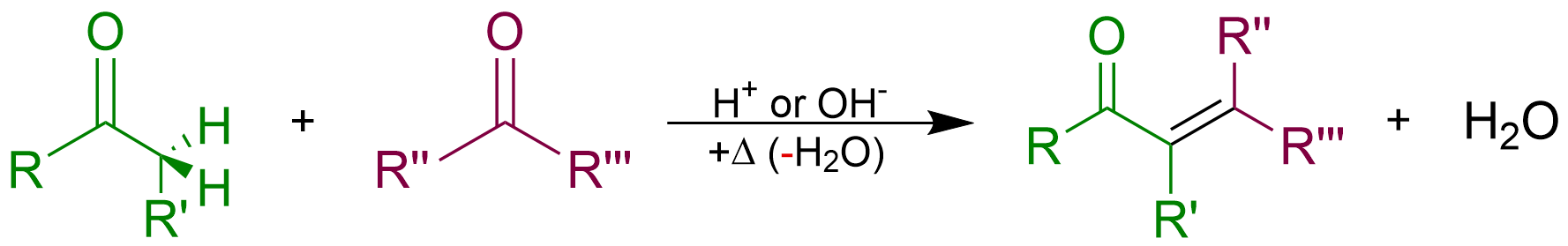|
Cinnamaldehyde Acsv
Cinnamaldehyde is an organic compound with the formula or . Occurring naturally as predominantly the Cis-trans isomerism, ''trans'' (''E'') isomer, it gives cinnamon its Flavoring, flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, Viscosity, viscous liquid occurs in the Bark (botany), bark of cinnamon trees and other species of the genus ''Cinnamomum''. It is an essential oil. The bark of cinnamon tree contains high concentrations of cinnamaldehyde. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugû´ne-Melchior Pûˋligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is ''Geometric isomerism, trans''-cinnamaldehyde. The molecule consists of a benzene ring attached to an unsaturated aldehyde. Cinnamaldehyde is an öÝ,öý-Unsaturated carbonyl compound, öÝ,öý-unsaturated carbonyl compound. Its color is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamon
Cinnamon is a spice obtained from the inner bark of several tree species from the genus ''Cinnamomum''. Cinnamon is used mainly as an aromatic condiment and flavouring additive in a wide variety of cuisines, sweet and savoury dishes, biscuits, breakfast cereals, Snack, snack foods, bagels, teas, hot chocolate and traditional foods. The aroma and flavour of cinnamon derive from its essential oil and principal component, cinnamaldehyde, as well as numerous other constituents, including eugenol. Cinnamon is the name for several species of trees and the commercial spice products that some of them produce. All are members of the genus ''Cinnamomum'' in the family Lauraceae. Only a few ''Cinnamomum'' species are grown commercially for spice. ''Cinnamomum verum'' (alternatively ''C. zeylanicum''), known as "Ceylon cinnamon" after its origins in Sri Lanka (formerly Ceylon), is considered to be "true cinnamon", but most cinnamon in international commerce is derived from four other speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Essential Oil
An essential oil is a concentrated hydrophobic liquid containing volatile (easily evaporated at normal temperatures) chemical compounds from plants. Essential oils are also known as volatile oils, ethereal oils, aetheroleum, or simply as the oil of the plant from which they were extracted, such as oil of clove. An essential oil is essential in the sense that it contains the essence of the plant's fragranceãthe characteristic fragrance of the plant from which it is derived. The term "essential" used here does ''not'' mean required or usable by the human body, as with the terms essential amino acid or essential fatty acid, which are so called because they are nutritionally required by a living organism. Essential oils are generally extracted by distillation, often by using steam. Other processes include expression, solvent extraction, '' sfumatura'', absolute oil extraction, resin tapping, wax embedding, and cold pressing. They are used in perfumes, cosmetics, soaps, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is among the simplest aromatic aldehydes and one of the most industrially useful. It is a colorless liquid with a characteristic almond-like odor, and is commonly used in cherry-flavored sodas. A component of bitter almond oil, benzaldehyde can be extracted from a number of other natural sources. Synthetic benzaldehyde is the flavoring agent in imitation almond extract, which is used to flavor cakes and other baked goods. History Benzaldehyde was first extracted in 1803 by the French pharmacist Martrû´s. His experiments focused on elucidating the nature of amygdalin, the poisonous compound found in bitter almonds, the fruit of '' Prunus dulcis''. Further work on the oil by Pierre Robiquet and Antoine Boutron Charlard, two French chemists, produced benzaldehyde. In 1832, Friedrich WûÑhler and Justus von Liebig first synthesized benzaldehyde. Production Benzaldeh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a öý-hydroxyaldehyde or öý-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction equation is as follows (where the Rs can be H) Aldol condensations are important in organic synthesis and biochemistry as ways to form carbonãcarbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a öý-hydroxy ketone, or aldol (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the processãthe aldol reaction itselfãas catalyzed by aldolases. However, the first step is formally an addition reaction rather than a condensation reaction bec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamyl Alcohol
Cinnamyl alcohol or styron is an organic compound that is found in esterified form in storax, Balsam of Peru, and cinnamon leaves. It forms a white crystalline solid when pure, or a yellow oil when even slightly impure. It can be produced by the hydrolysis of storax. Cinnamyl alcohol occurs naturally only in small quantities, so its industrial demand is usually fulfilled by chemical synthesis starting from cinnamaldehyde. Properties The compound is a solid at room temperature, forming colorless crystals that melt upon gentle heating. As is typical of most higher-molecular weight alcohols, it is sparingly soluble in water at room temperature, but highly soluble in most common organic solvents. Uses Cinnamyl alcohol has a distinctive odor described as "sweet, balsam, hyacinth, spicy, green, powdery, cinnamic" and is used in perfumery and as a deodorant. Cinnamyl alcohol is the starting material used in the synthesis of reboxetine. Safety Cinnamyl alcohol has been found to have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic, cabbage or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the smell of natural gas is due to the smell of the thiol used as the odorant. Nomenclature Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
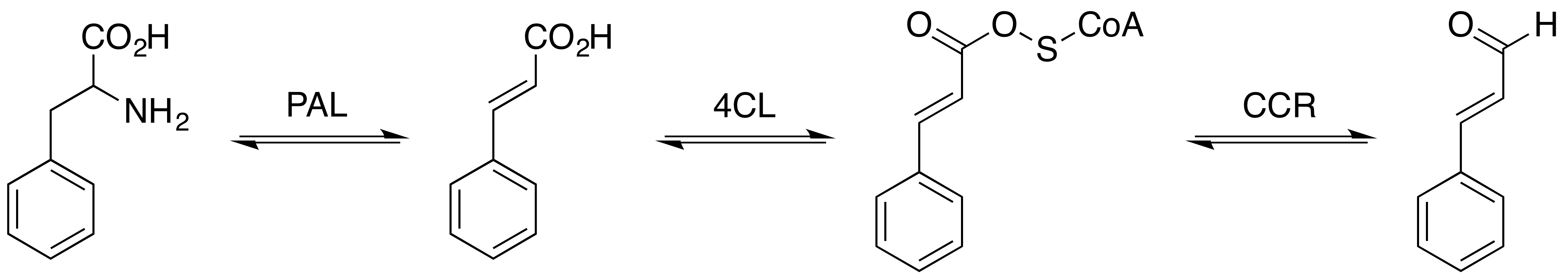
Phenylalanine Ammonia Lyase
The enzyme phenylalanine ammonia lyase (EC 4.3.1.24) catalyzes the conversion of L-phenylalanine to ammonia and ''trans''-cinnamic acid.: :L-phenylalanine = ''trans''-cinnamate + NH3 Phenylalanine ammonia lyase (PAL) is the first and committed step in the phenyl propanoid pathway and is therefore involved in the biosynthesis of the polyphenol compounds such as flavonoids, phenylpropanoids, and lignin in plants. Phenylalanine ammonia lyase is found widely in plants, as well as some bacteria, yeast, and fungi, with isoenzymes existing within many different species. It has a molecular mass in the range of 270ã330 kDa. The activity of PAL is induced dramatically in response to various stimuli such as tissue wounding, pathogenic attack, light, low temperatures, and hormones. PAL has recently been studied for possible therapeutic benefits in humans afflicted with phenylketonuria. It has also been used in the generation of L-phenylalanine as precursor of the sweetener aspar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamic Acid
Cinnamic acid is an organic compound with the formula phenyl, C6H5-CH=CH-Carboxylic acid, COOH. It is a white crystalline compound that is slightly soluble in water, and freely soluble in many organic solvents. Classified as an unsaturated carboxylic acid, it occurs naturally in a number of plants. It exists as both a Cisãtrans isomerism, ''cis'' and a ''trans'' isomer, although the latter is more common. The ''cis''-isomer is called allocinnamic acid. Occurrence and production Biosynthesis Cinnamic acid is a central intermediate in the biosynthesis of a myriad of natural products including lignols (precursors to lignin and lignocellulose), flavonoids, isoflavonoids, coumarins, aurones, stilbenes, catechin, and phenylpropanoids. Its biosynthesis involves the action of the enzyme phenylalanine ammonia-lyase (PAL) on phenylalanine. Natural occurrence It is obtained from oil of cinnamon, or from balsams such as storax. It is also found in shea butter. Cinnamic acid has a honey-like ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deamination
Deamination is the removal of an amino group from a molecule. Enzymes that catalysis, catalyse this reaction are called deaminases. In the human body, deamination takes place primarily in the liver; however, it can also occur in the kidney. In situations of excess protein intake, deamination is used to break down amino acids for energy. The amino group is removed from the amino acid and converted to ammonia. The rest of the amino acid is made up of mostly carbon and hydrogen, and is recycled or oxidized for energy. Ammonia is toxic to the human system, and enzymes convert it to urea or uric acid by addition of carbon dioxide molecules (which is not considered a deamination process) in the urea cycle, which also takes place in the liver. Urea and uric acid can safely diffuse into the blood and then be excreted in urine. Deamination reactions in DNA Cytosine Spontaneous deamination is the hydrolysis reaction of cytosine into uracil, releasing ammonia in the process. This can occu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine
Phenylalanine (symbol Phe or F) is an essential öÝ-amino acid with the chemical formula, formula . It can be viewed as a benzyl group substituent, substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and chemical polarity, nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The chirality (chemistry)#Naming conventions, L-isomer is used to biochemically form proteins coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the biological pigment melanin. It is Genetic code, encoded by the messenger RNA codons UUU and UUC. Phenylalanine is found naturally in the milk of mammals. It is used in the manufacture of food and drink products and sold as a nutritional supplement as it is a direct precursor to the neuromodulation, neuromodulator phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cinnamaldehyde Biosynthesis Pathway
Cinnamaldehyde is an organic compound with the formula or . Occurring naturally as predominantly the Cis-trans isomerism, ''trans'' (''E'') isomer, it gives cinnamon its Flavoring, flavor and odor. It is a phenylpropanoid that is naturally synthesized by the shikimate pathway. This pale yellow, Viscosity, viscous liquid occurs in the Bark (botany), bark of cinnamon trees and other species of the genus ''Cinnamomum''. It is an essential oil. The bark of cinnamon tree contains high concentrations of cinnamaldehyde. Structure and synthesis Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugû´ne-Melchior Pûˋligot and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854. The natural product is ''Geometric isomerism, trans''-cinnamaldehyde. The molecule consists of a benzene ring attached to an unsaturated aldehyde. Cinnamaldehyde is an öÝ,öý-Unsaturated carbonyl compound, öÝ,öý-unsaturated carbonyl compound. Its color is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
öÝ,öý-Unsaturated Carbonyl Compound
öÝ,öý-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)ãCöÝ=CöýãR. Such compounds include enones and enals, but also carboxylic acids and the corresponding esters and amides. In these compounds, the carbonyl group is conjugated with an alkene (hence the adjective unsaturated). Unlike the case for carbonyls without a flanking alkene group, öÝ,öý-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the öý-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction. Classifications öÝ,öý-Unsaturated carbonyl compounds can be subclassified according to the nature of the carbonyl and alkene groups. File:Methyl vinyl ketone.svg, Methyl vinyl ketone, the simplest öÝ,öý-unsaturated ketone File:Acrolein-2D-skeletal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |