|
Chromium(II) Iodide
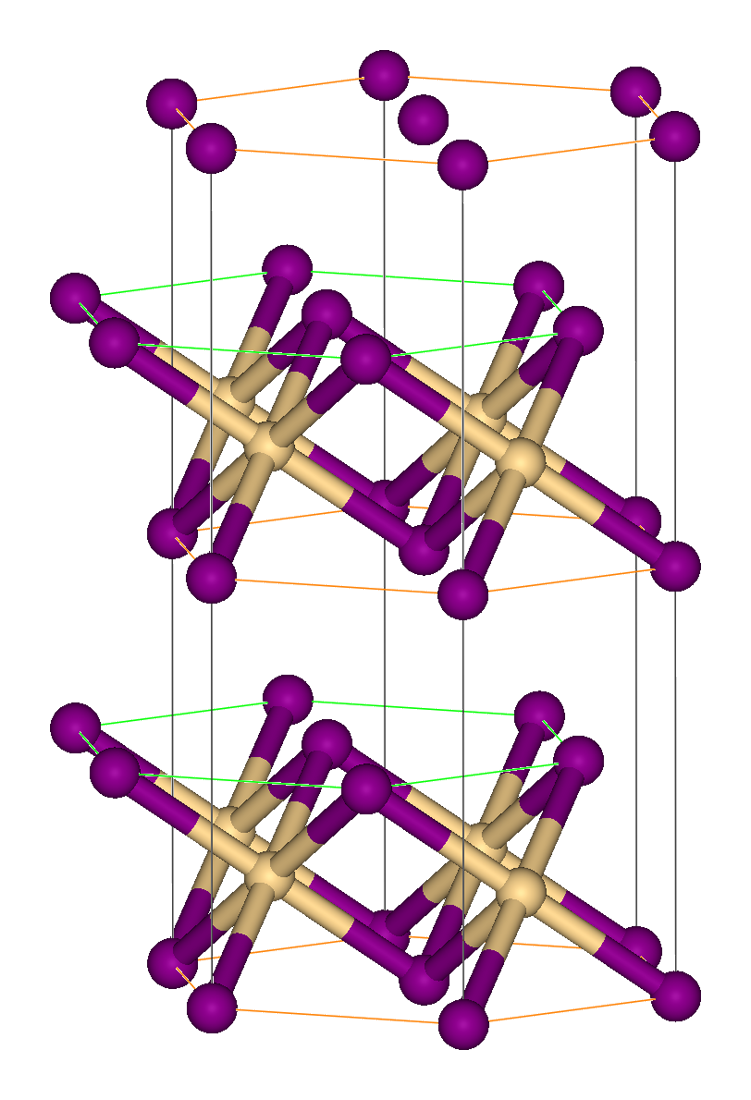
Chromium(II) iodide is the inorganic compound with the formula CrI2. It is a red-brown or black solid. The compound is made by thermal decomposition of chromium(III) iodide. Like many metal diiodides, CrI2 adopts the "cadmium iodide structure" motif, i.e., it features sheets of octahedral Cr(II) centers interconnected by bridging iodide ligands. Reflecting the effects of its d4 configuration, chromium's coordination sphere is highly distorted. Treatment of chromium powder with concentrated hydroiodic acid gives a blue hydrated chromium(II) iodide, which can be converted to related acetonitrile complex Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile. Scope of nitriles Typical nitrile ligands are acetonitrile, pro ...es. :Cr + nH2O + 2HI → CrI2(H2O)n + H2 References {{Chromium compounds Chromium(II) compounds Iodides Metal hali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon ( graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Decomposition
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction. Decomposition temperature definition A simple substance (like water) may exist in equilibrium with its thermal decomposition products, effectively halting the decomposition. The equilibrium fraction of decomposed molecules increases with the temperature. Examples * Calcium carbonate (limestone or chalk) decomposes into calcium oxide and carbon dioxide when heated. The chemical reaction is as follows: ::CaCO3 → CaO + CO2 :The reaction is used to make quick lime, which is an industrially important product. :A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(III) Iodide
Chromium(III) iodide, also known as chromium triiodide, is an inorganic compound with the formula . It is a black solid that is used to prepare other chromium iodides. Like the isomorphous chromium(III) chloride (), chromium(III) iodide exhibits a cubic-closest packing arrangement in a double-layer crystal lattice. In this structure, chromium exhibits octahedral coordination geometry. Preparation and properties Chromium triiodide is prepared by the direct reaction of chromium metal with an excess of iodine. The reaction is conducted at 500 °C: : To obtain high purity samples, the product is thermally decomposed at 700 °C to sublime out chromium(II) iodide. The diiodide is then reiodinated. Chromium triiodide is stable in contact with oxygen and moisture, but at temperatures approaching 200 °C it reacts with oxygen and releases iodine. Like , the triiodide exhibits slow solubility in water owing to the kinetic inertness of Cr(III). Addition of small amounts of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Iodide
Cadmium iodide is the inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It has few applications. It is notable for its crystal structure, which is typical for compounds of the form MX2 with strong polarization effects. Preparation Cadmium iodide is prepared by the addition of cadmium metal, or its oxide, hydroxide or carbonate to hydroiodic acid. Also, the compound can be made by heating cadmium with iodine. Crystal structure In cadmium iodide the iodide anions form a hexagonal close packed arrangement while the cadmium cations fill all of the octahedral sites in alternate layers. The resultant structure consists of a layered lattice. This same basic structure is found in many other salts and minerals. Cadmium iodide is mostly ionically bonded but with partial covalent character. Cadmium iodide's crystal structure is the prototype on which the crystal structures many other compounds can be conside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jahn–Teller Effect
The Jahn–Teller effect (JT effect or JTE) is an important mechanism of spontaneous symmetry breaking in molecular and solid-state systems which has far-reaching consequences in different fields, and is responsible for a variety of phenomena in spectroscopy, stereochemistry, crystal chemistry, molecular and solid-state physics, and materials science. The effect is named for Hermann Arthur Jahn and Edward Teller, who first reported studies about it in 1937.Bunker, Philip R.; Jensen, Per (1998) ''Molecular Symmetry and Spectroscopy'' (2nd ed.). NRC Research Press, Ottaw/ref> Simplified overview The Jahn–Teller effect, sometimes also referred to as Jahn–Teller distortion, describes the geometrical distortion of molecules and ions that result from certain electron configurations. The Jahn–Teller theorem essentially states that any non-linear molecule with a spatially degenerate electronic ground state will undergo a geometrical distortion that removes that degeneracy, because t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
D Electron Count
The d electron count is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition metal center in a coordination complex. The d electron count is an effective way to understand the geometry and reactivity of transition metal complexes. The formalism has been incorporated into the two major models used to describe coordination complexes; crystal field theory and ligand field theory, which is a more advanced version based on molecular orbital theory. Standard electron configuration perspective The electron configuration for transition metals predicted by the simple Aufbau principle and Madelung's rule has serious conflicts with experimental observations for transition metal centers under most ambient conditions. Under most conditions all of the valence electrons of a transition metal center are located in d orbitals while the standard model of electron configuration would predict some of them to be in the pertinent s orbital. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroiodic Acid
Hydroiodic acid (or hydriodic acid) is an aqueous solution of hydrogen iodide (HI). It is a strong acid, one that is ionized completely in an aqueous solution. It is colorless. Concentrated solutions are usually 48% to 57% HI. Reactions Hydroiodic acid reacts with oxygen in air to give iodine: :4 HI + O2 → 2 + 2 I2 Like other hydrogen halides, hydroiodic acid adds to alkenes to give alkyl iodides. It can also be used as a reducing agent, for example in the reduction of aromatic nitro compounds to anilines. Cativa process The Cativa process is a major end use of hydroiodic acid, which serves as a co-catalyst for the production of acetic acid by the carbonylation of methanol. Illicit uses Hydroiodic acid is listed as a U.S. Federal DEA List I Chemical, owing to its use as a reducing agent related to the production of methamphetamine from ephedrine or pseudoephedrine Pseudoephedrine (PSE) is a sympathomimetic drug of the phenethylamine and amphetamine chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetonitrile Complex
Transition metal nitrile complexes are coordination compounds containing nitrile ligands. Because nitriles are weakly basic, the nitrile ligands in these complexes are often labile. Scope of nitriles Typical nitrile ligands are acetonitrile, propionitrile, and benzonitrile. The structures of u(NH3)5(NCPh)sup>n+ have been determined for the 2+ and 3+ oxidation states. Upon oxidation the Ru-NH3 distances contract and the Ru-NCPh distances elongate, consistent with amines serving as pure-sigma donor ligands and nitriles functioning as pi-acceptors. Synthesis and reactions Acetonitrile, propionitrile and benzonitrile are also popular solvents. Because nitrile solvents have high dielectric constants, cationic complexes containing a nitrile ligand are often soluble in a solution of that nitrile. Some complexes can be prepared by dissolving an anhydrous metal salt in the nitrile. In other cases, a suspension of the metal is oxidized with a solution of NOBF4 in the nitrile: :Ni + 6 M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromium(II) Compounds
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal. Chromium metal is valued for its high corrosion resistance and hardness. A major development in steel production was the discovery that steel could be made highly resistant to corrosion and discoloration by adding metallic chromium to form stainless steel. Stainless steel and chrome plating (electroplating with chromium) together comprise 85% of the commercial use. Chromium is also greatly valued as a metal that is able to be highly polished while resisting tarnishing. Polished chromium reflects almost 70% of the visible spectrum, and almost 90% of infrared light. The name of the element is derived from the Greek word χρῶμα, ''chrōma'', meaning color, because many chromium compounds are intensely colored. Industrial production of chromium proceeds from chromite ore (mostly FeCr2O4) to produce fe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodides
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency affects two billion people and is the leading preventable cause of intellectual disability. Structure and characteristics of inorganic iodides Iodide is one of the largest monatomic anions. It is assigned a radius of around 206 picometers. For comparison, the lighter halides are considerably smaller: bromide (196 pm), chloride (181 pm), and fluoride (133 pm). In part because of its size, iodide forms relatively weak bonds with most elements. Most iodide salts are soluble in water, but often less so than the related chlorides and bromides. Iodide, being large, is less hydrophilic compared to the smaller anions. One consequence of this is that sodium iodide is highly soluble in acetone, whereas sodium chloride is n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-3D-balls.png)
.png)

copper(I)_hexafluorophosphate.png)
