|
Channel-iron Deposits
Channel iron deposits (CID) are iron-rich fluvial sedimentary deposits of possible Miocene age occupying meandering palaeochannels in the Early to Mid-Cenozoic Hamerlsey palaeosurface of Western Australia. Examples are also known from Kazakhstan. The deposits are anomalously high in iron for detrital material, and exclude detrital iron deposits typified by scree of hematitic banded iron formations and accumulations of currently-forming maghemite pisolite alluvials. CIDs are a major source of cheap, high grade iron ore exploited primarily in the Pilbara and Murchison regions of Western Australia. Morphology Channel iron deposits are typically partly eroded and currently are from between 5 km. Mineralised channels are up to 150 kilometres in length, but not all of the preserved length of the CID is of ore grade. Channel iron systems typically form within a depression on the Cenozoic ‘Hamersley Surface’, and form several pods downstream on the palaeodrainage. The channe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's outer and inner core. It is the fourth most abundant element in the Earth's crust, being mainly deposited by meteorites in its metallic state. Extracting usable metal from iron ores requires kilns or furnaces capable of reaching , about 500 °C (900 °F) higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BC and the use of iron tools and weapons began to displace copper alloys – in some regions, only around 1200 BC. That event is considered the transition from the Bronze Age to the Iron Age. In the modern world, iron alloys, such as steel, stainless steel, cast iron and special steels, are by far the most common industrial metals, due to their mechan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Humic
Humic substances (HS) are colored relatively recalcitrant organic compounds naturally formed during long-term decomposition and transformation of biomass residues. The color of humic substances varies from bright yellow to light or dark brown leading to black. The term comes from humus, which in turn comes from the Latin word ''humus'', meaning "soil, earth". Humic substances represent the major part of organic matter in soil, peat, coal, and sediments, and are important components of dissolved natural organic matter (NOM) in lakes (especially dystrophic lakes), rivers, and sea water. Humic substances account for 50 – 90% of cation exchange capacity in soils. "Humic substances" is an umbrella term covering humic acid, fulvic acid and humin, which differ in solubility. By definition, humic acid (HA) is soluble in water at neutral and alkaline pH, but insoluble at acidic pH < 2. Fulvic acid (FA) is soluble in water at any pH. Humin is not soluble in water at any pH. This definit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows Tetrahedral molecular geometry, tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siderite
Siderite is a mineral composed of iron(II) carbonate (FeCO3). Its name comes from the Ancient Greek word (), meaning "iron". A valuable iron ore, it consists of 48% iron and lacks sulfur and phosphorus. Zinc, magnesium, and manganese commonly substitute for the iron, resulting in the siderite-smithsonite, siderite-magnesite, and siderite-rhodochrosite solid solution series. Siderite has Mohs hardness of 3.75 to 4.25, a specific gravity of 3.96, a white streak and a vitreous or pearly luster. Siderite is antiferromagnetic below its Néel temperature of that can assist in its identification. It crystallizes in the trigonal crystal system; crystals are rhombohedral in shape, typically with curved and striated faces. It also occurs in masses. Color ranges from yellow to dark brown or black, the latter being due to the presence of manganese. Siderite is commonly found in hydrothermal veins, and is associated with barite, fluorite, galena, and others. It is also a common ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcite
Calcite is a Carbonate minerals, carbonate mineral and the most stable Polymorphism (materials science), polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of limestone. Calcite defines hardness 3 on the Mohs scale of mineral hardness, based on Scratch hardness, scratch hardness comparison. Large calcite crystals are used in optical equipment, and limestone composed mostly of calcite has numerous uses. Other polymorphs of calcium carbonate are the minerals aragonite and vaterite. Aragonite will change to calcite over timescales of days or less at temperatures exceeding 300 °C, and vaterite is even less stable. Etymology Calcite is derived from the German , a term from the 19th century that came from the Latin word for Lime (material), lime, (genitive ) with the suffix ''-ite'' used to name minerals. It is thus a Doublet (linguistics), doublet of the word ''wikt:chalk, chalk''. When applied by archaeology, archaeologists and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesite
Magnesite is a mineral with the chemical formula ( magnesium carbonate). Iron, manganese, cobalt, and nickel may occur as admixtures, but only in small amounts. Occurrence Magnesite occurs as veins in and an alteration product of ultramafic rocks, serpentinite and other magnesium rich rock types in both contact and regional metamorphic terrains. These magnesites are often cryptocrystalline and contain silica in the form of opal or chert. Magnesite is also present within the regolith above ultramafic rocks as a secondary carbonate within soil and subsoil, where it is deposited as a consequence of dissolution of magnesium-bearing minerals by carbon dioxide in groundwaters. Formation Magnesite can be formed via talc carbonate metasomatism of peridotite and other ultramafic rocks. Magnesite is formed via carbonation of olivine in the presence of water and carbon dioxide at elevated temperatures and high pressures typical of the greenschist facies. Magnesite can also be f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonate Mineral
Carbonate minerals are those minerals containing the carbonate ion, . Carbonate divisions Anhydrous carbonates *Calcite group: trigonal **Calcite CaCO3 **Gaspéite (Ni,Mg,Fe2+)CO3 **Magnesite MgCO3 **Otavite CdCO3 **Rhodochrosite MnCO3 **Siderite FeCO3 **Smithsonite ZnCO3 **Spherocobaltite CoCO3 *Aragonite group: orthorhombic **Aragonite CaCO3 **Cerussite PbCO3 **Strontianite SrCO3 **Witherite BaCO3 **Rutherfordine UO2CO3 **Natrite Na2CO3 Anhydrous carbonates with compound formulas *Dolomite group: trigonal **Ankerite CaFe(CO3)2 **Dolomite (mineral), Dolomite CaMg(CO3)2 **Huntite Mg3Ca(CO3)4 **Minrecordite CaZn(CO3)2 **Barytocalcite BaCa(CO3)2 Carbonates with hydroxyl or halogen *Carbonate with hydroxide: monoclinic **Azurite Cu3(CO3)2(OH)2 **Hydrocerussite Pb3(CO3)2(OH)2 **Malachite Cu2CO3(OH)2 **Rosasite (Cu,Zn)2CO3(OH)2 **Phosgenite Pb2(CO3)Cl2 **Hydrozincite Zn5(CO3)2(OH)6 **Aurichalcite (Zn,Cu)5(CO3)2(OH)6 Hydrated carbonates *Hydromagnesite Mg5(CO3)4(OH)2.4H2O *Ikaite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clay Mineral
Clay minerals are hydrous aluminium phyllosilicates (e.g. kaolin, Al2 Si2 O5( OH)4), sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations found on or near some planetary surfaces. Clay minerals form in the presence of water and have been important to life, and many theories of abiogenesis involve them. They are important constituents of soils, and have been useful to humans since ancient times in agriculture and manufacturing. Properties Clay is a very fine-grained geologic material that develops plasticity when wet, but becomes hard, brittle and non–plastic upon drying or firing. It is a very common material, and is the oldest known ceramic. Prehistoric humans discovered the useful properties of clay and used it for making pottery. The chemistry of clay, including its capacity to retain nutrient cations such as potassium and ammonium, is important to soil fertility. Because the individual particles in clay are less ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Limonite
Limonite () is an iron ore consisting of a mixture of hydrated iron(III) oxide-hydroxides in varying composition. The generic formula is frequently written as , although this is not entirely accurate as the ratio of oxide to hydroxide can vary quite widely. Limonite is one of the three principal iron ores, the others being hematite and magnetite, and has been mining, mined for the production of iron since at least 400 BC. Names Limonite is named for the Ancient Greek word ( ), meaning "wet meadow", or ( ), meaning "marshy lake", as an allusion to its occurrence as in meadows and marshes. In its brown form, it is sometimes called brown hematite or brown iron ore. Characteristics Limonite is relatively density, dense with a specific gravity varying from 2.7 to 4.3.Northrop, Stuart A. (1959) "Limonite" ''Minerals of New Mexico'' (revised edition) University of New Mexico Press, Albuquerque, New Mexico, pp. 329–333, It is usually medium to dark yellowish brown in color. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Goethite
Goethite (, ) is a mineral of the diaspore group, consisting of iron(III) oxide-hydroxide, specifically the α- polymorph. It is found in soil and other low-temperature environments such as sediment. Goethite has been well known since ancient times for its use as a pigment (brown ochre). Evidence has been found of its use in paint pigment samples taken from the caves of Lascaux in France. It was first described in 1806 based on samples found in the Hollertszug Mine in Herdorf, Germany. The mineral was named after the German polymath and poet Johann Wolfgang von Goethe (1749–1832). Composition Goethite is an iron oxyhydroxide containing ferric iron. It is the main component of rust and bog iron ore. Goethite's hardness ranges from 5.0 to 5.5 on the Mohs Scale, and its specific gravity varies from 3.3 to 4.3. The mineral forms prismatic needle-like crystals ("needle ironstone") but is more typically massive. Feroxyhyte and lepidocrocite are both polymorphs of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
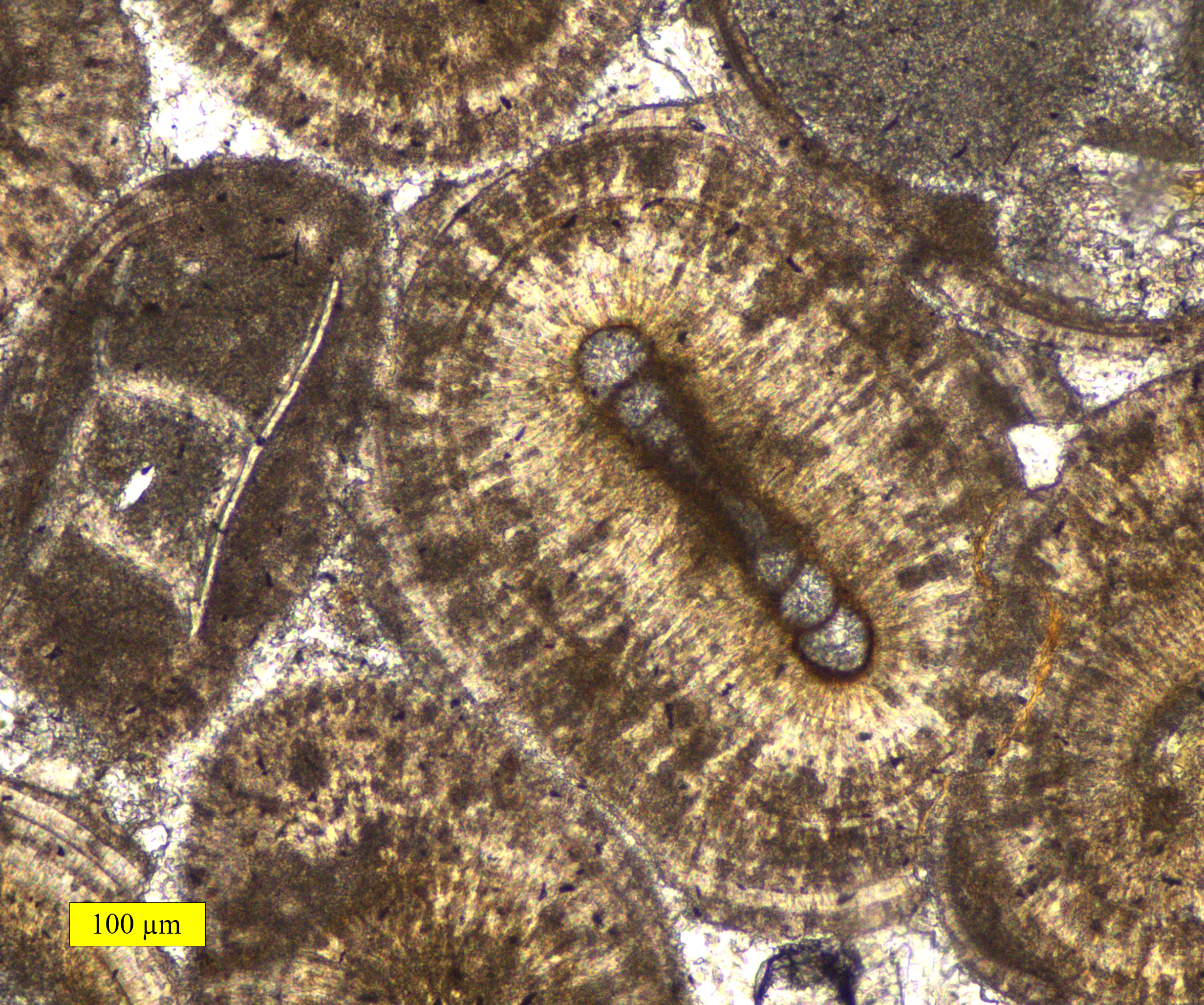
Pisoid
A pisolite () is a sedimentary rock made of pisoids, which are concretionary grains – typically of calcium carbonate which resemble ooids, but are more than 2 mm in diameter. These grains are approximately spherical and have concentric layers reaching 10 mm in diameter. Bauxites, limonites, and siderites often have a pisolitic structure. See also * Ooid * Oolite Oolite or oölite () is a sedimentary rock formed from ooids, spherical grains composed of concentric layers. Strictly, oolites consist of ooids of diameter 0.25–2 millimetres; rocks composed of ooids larger than 2 mm are called pis ... References Further reading * Sedimentary rocks {{sedimentary-rock-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ooid
Ooids (, ) are small (commonly ≤2 mm in diameter), spheroidal, "coated" (layered) sedimentary grains, usually composed of calcium carbonate, but sometimes made up of iron- or phosphate-based minerals. Ooids usually form on the sea floor, most commonly in shallow tropical seas (around the Bahamas, for example, or in the Persian Gulf). After being buried under additional sediment, these ooid grains can be cemented together to form a sedimentary rock called an '' oolite''. Oolites usually consist of calcium carbonate; these belong to the limestone rock family. Pisoids are similar to ooids, but are larger than 2 mm in diameter, often considerably larger, as with the pisoids in the hot springs at Carlsbad (Karlovy Vary) in the Czech Republic. Ooids have been the subject of scientific research for centuries.https://www.geological-digressions.com/the-mineralogy-of-carbonates-non-skeletal-grains/ Formation An ooid forms as a series of concentric layers around a nucleus. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |