|
Castro–Stephens Coupling
The Castro–Stephens coupling is a cross coupling reaction between a copper(I) acetylide and an aryl halide in pyridine, forming a disubstituted alkyne and a copper(I) halide. : The reaction was described in 1963 by chemists Castro and Stephens. The reaction is similar to the much older Rosenmund–von Braun synthesis (1914) between aryl halides and copper(I) cyanide and was itself modified in 1975 as the Sonogashira coupling by adding a palladium catalyst and preparing the organocopper compound ''in situ'', allowing copper to also be used catalytically. A typical reaction diphenylacetylene is obtained by the coupling of iodobenzene with Cuphenylacetylene, C2C6H5 in hot pyridine: : Unlike the Sonogashira coupling, the Castro–Stephens coupling can produce heterocyclic compounds when a nucleophilic group is ''arene substitution pattern, ortho'' to the aryl halide, although this typically requires use of dimethylformamide (DMF) as solvent. : References {{DEFAULTSORT:Cas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
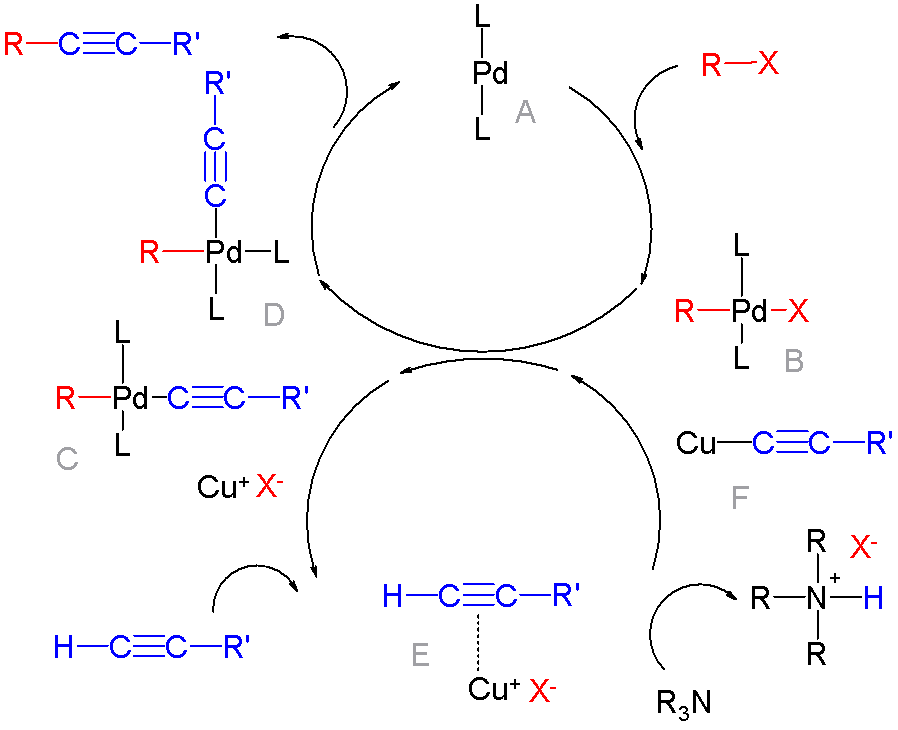
Cross Coupling Reaction
In organic chemistry, a cross-coupling reaction is a reaction where two different fragments are joined. Cross-couplings are a subset of the more general coupling reactions. Often cross-coupling reactions require metal catalysts. One important reaction type is this: : (R, R' = organic fragments, usually aryl; M = main group center such as Li or MgX; X = halide) These reactions are used to form carbon–carbon bonds but also carbon-heteroatom bonds. Cross-coupling reaction are a subset of coupling reactions. Richard F. Heck, Ei-ichi Negishi, and Akira Suzuki were awarded the 2010 Nobel Prize in Chemistry for developing palladium-catalyzed coupling reactions. Mechanism Many mechanisms exist reflecting the myriad types of cross-couplings, including those that do not require metal catalysts. Often, however, cross-coupling refers to a metal-catalyzed reaction of a nucleophilic partner with an electrophilic partner. In such cases, the mechanism generally involves reductive elimin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
In Situ
is a Latin phrase meaning 'in place' or 'on site', derived from ' ('in') and ' ( ablative of ''situs'', ). The term typically refers to the examination or occurrence of a process within its original context, without relocation. The term is used across many disciplines to denote methods, observations, or interventions carried out in their natural or intended environment. By contrast, ' methods involve the removal or displacement of materials, specimens, or processes for study, preservation, or modification in a controlled setting, often at the cost of contextual integrity. The earliest known use of ''in situ'' in the English language dates back to the mid-17th century. In scientific literature, its usage increased from the late 19th century onward, initially in medicine and engineering. The natural sciences typically use methods to study phenomena in their original context. In geology, field analysis of soil composition and rock formations provides direct insights into Earth' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylformamide
Dimethylformamide, DMF is an organic compound with the chemical formula . Its structure is . Commonly abbreviated as DMF (although this initialism is sometimes used for 2,5-dimethylfuran, dimethylfuran, or dimethyl fumarate), this colourless liquid is Miscibility, miscible with Water (molecule), water and the majority of organic liquids. DMF is a common solvent for chemical reactions. Dimethylformamide is odorless, but chemical purity, technical-grade or degraded samples often have a fishy smell due to impurity of dimethylamine. Dimethylamine degradation impurities can be removed by Sparging (chemistry), sparging samples with an inert gas such as argon or by sonication, sonicating the samples under reduced pressure. As its name indicates, it is structurally related to formamide, having two methyl groups in the place of the two hydrogens. DMF is a polar molecule, polar (hydrophilic) aprotic solvent with a high boiling point. It facilitates reactions that follow polar mechanisms, such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arene Substitution Pattern
Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon. ''Ortho'', ''meta'', and ''para'' substitution * In ''ortho''-substitution, two substituents occupy positions next to each other, which may be numbered 1 and 2. In the diagram, these positions are marked R and ''ortho''. * In ''meta''-substitution, the substituents occupy positions 1 and 3 (corresponding to R and ''meta'' in the diagram). * In ''para''-substitution, the substituents occupy the opposite ends (positions 1 and 4, corresponding to R and ''para'' in the diagram). The toluidines serve as an example for these three types of substitution. Synthesis Electron donating groups, for example amino, hydroxyl, alkyl, and phenyl groups tend to be ''ortho''/''para''-directors, and electron withdrawing groups such as nitro, nitrile, and ketone groups, tend to be ''meta''-directors. Pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylacetylene
Phenylacetylene is an alkyne hydrocarbon containing a phenyl group. It exists as a colorless, viscous liquid. In research, it is sometimes used as an analog for acetylene; being a liquid, it is easier to handle than acetylene gas. Preparation In the laboratory, phenylacetylene can be prepared by elimination of hydrogen bromide from styrene dibromide using sodium amide in ammonia: : It can also be prepared by the elimination of hydrogen bromide from bromostyrene using molten potassium hydroxide. Yet another method involves the Sonogashira coupling of iodobenzene with trimethylsilylacetylene, followed by removal of the trimethylsilyl group using TBAF. Reactions Phenylacetylene is a prototypical terminal acetylene, undergoing many reactions expected of that functional group. It undergoes semihydrogenation over Lindlar catalyst to give styrene. In the presence of base and copper(II) salts, it undergoes oxidative coupling to give diphenylbutadiyne. In the presence of metal cata ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodobenzene
Iodobenzene is an aryl iodide and the simplest of the iodobenzenes, consisting of a benzene ring substituted with one iodine atom. Its chemical formula is . It is useful as a synthetic intermediate in organic chemistry. It is a volatile colorless liquid, although aged samples appear yellowish. Preparation Iodobenzene is commercially available, or it can be prepared in the laboratory from aniline via the diazotization reaction. In the first step, the amine functional group is diazotized with hydrochloric acid and sodium nitrite. Potassium iodide is added to the resultant phenyldiazonium chloride, causing nitrogen gas to evolve. The product is separated by steam distillation. : Alternatively, it can be produced by refluxing iodine and nitric acid with benzene. Reactions Since the C–I bond is weaker than C–Br or C–Cl, iodobenzene is more reactive than bromobenzene or chlorobenzene. Iodobenzene reacts readily with magnesium to form the Grignard reagent, phenylmagnesium i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diphenylacetylene
Diphenylacetylene is the chemical compound C6H5C≡CC6H5. The molecule consists of two phenyl groups attached to a C2 unit. A colorless solid, it is used as a building block in organic synthesis and as a Transition metal alkyne complex, ligand in organometallic chemistry. Preparation and structure In one preparation for this compound, benzil is condensed with hydrazine to give the bis(hydrazone), which is oxidized with mercury(II) oxide. Alternatively stilbene is brominated, and the resulting dibromodiphenylethane is subjected to dehydrohalogenation. Yet another method involves the coupling of iodobenzene and the copper salt of phenylacetylene in the Castro-Stephens coupling. The related Sonogashira coupling involves the coupling of iodobenzene and phenylacetylene. Diphenylacetylene is a planar molecule. The central C≡C distance is 119.8 picometers. Derivatives 1,2,3,4-Tetraphenylbutadiene is prepared by reductive coupling of diphenylacetylene using lithium metal followed by h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedron Lett
''Tetrahedron Letters'' is a weekly international journal for rapid publication of full original research papers in the field of organic chemistry. According to the ''Journal Citation Reports'', the journal has a 2022 impact factor of 1.8 Indexing ''Tetrahedron Letters'' is indexed in: References See also *''Tetrahedron In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...'' *'' Tetrahedron: Asymmetry'' Chemistry journals Weekly journals Academic journals established in 1959 Elsevier academic journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organocopper Compound
Organocopper chemistry is the study of the physical properties, reactions, and synthesis of organocopper compounds, which are organometallic compounds containing a carbon to copper chemical bond. They are reagents in organic chemistry. The first organocopper compound, the explosive copper(I) acetylide (), was synthesized by Rudolf Christian Böttger in 1859 by passing acetylene gas through a solution of copper(I) chloride: : Structure and bonding Organocopper compounds are diverse in structure and reactivity, but almost all are based on copper with an oxidation state of +1, sometimes denoted Cu(I) or . With 10 electrons in its valence shell, the bonding behavior of Cu(I) is similar to Ni(0), but owing to its higher oxidation state, it engages in less pi-backbonding. Organic derivatives of copper's higher oxidation states of +2 and +3 are sometimes encountered as reaction intermediates, but rarely isolated or even observed. Organocopper compounds form complexes with a va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Acetylide
Copper(I) acetylide, copper carbide or cuprous acetylide, is a chemical compound with the formula . It is a copper(I) salt of acetylene. It consists of cations and acetylide anions , with the triple bond between the two carbon atoms. Although never characterized by X-ray crystallography, the material has been claimed at least since 1856. One form is claimed to be a monohydrate with formula . Copper(I) acetylide is a reddish-brown explosive powder. Synthesis Materials purported to be copper acetylide can be prepared by treating acetylene with a solution of copper(I) chloride and ammonia: : This reaction produces a reddish solid precipitate. Properties When dry, copper acetylide is a heat and shock sensitive primary explosive, more sensitive than silver acetylide. In acetylene manufacturing plants, copper acetylide is thought to form inside pipes made of copper or an alloy with high copper content, which may result in violent explosion. This led to abandonment of copper as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |