|
Calbindin
Calbindins are three different calcium-binding proteins: calbindin 1, calbindin, calretinin and S100G. They were originally described as vitamin D-dependent calcium-binding proteins in the intestine and kidney of chicks and mammals. They are now classified in different protein family, subfamilies as they differ in the number of Ca2+ binding EF hands. Calbindin 1 Calbindin 1 or simply calbindin was first shown to be present in the intestine in birds and then found in the mammalian kidney. It is also expressed in a number of neuronal and endocrine cells, particularly in the cerebellum. It is a 28 kDa protein encoded in humans by the ''CALB1'' gene. Calbindin contains 4 active calcium-binding protein domain, domains, and 2 modified domains that have lost their calcium-binding capacity. Calbindin acts as a calcium buffer and calcium sensor and can hold four Ca2+ in the EF-hands of loops EF1, EF3, EF4 and EF5. The structure of rat calbindin was originally solved by nuclear magnetic r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calbindin 1
Calbindin 1 is a protein that in humans is encoded by the ''CALB1'' gene. It belongs to the calbindin protein family, family of calcium-binding proteins, along with calretinin (''CALB2''). Function The protein encoded by this gene is a member of the calcium-binding protein superfamily that includes calmodulin and troponin C. Originally described as a 27 kDa protein, it is now known to be a 28 kDa protein. It contains four active calcium-binding domains, and has two modified domains that are thought to have lost their calcium binding capability. This protein is thought to buffer entry of calcium upon stimulation of glutamate receptors. Depletion of this protein was noted in patients with Huntington disease. [provided by RefSeq, Jan 2015]. References Further reading * * * * * * * * * {{gene-8-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
S100G
S100 calcium-binding protein G (S100G) is a protein that in humans is encoded by the ''S100G'' gene. This gene encodes calbindin Calbindins are three different calcium-binding proteins: calbindin 1, calbindin, calretinin and S100G. They were originally described as vitamin D-dependent calcium-binding proteins in the intestine and kidney of chicks and mammals. They are now ... D9K, a vitamin D-dependent calcium-binding protein. This cytosolic protein belongs to a family of calcium-binding proteins that includes calmodulin, parvalbumin, troponin C, and S100 protein. In the intestine, the protein is vitamin D-dependent and its expression correlates with calcium transport activity. The protein may increase Ca2+ absorption by buffering Ca2+ in the cytoplasm and increase ATP-dependent Ca2+ transport in duodenal basolateral membrane vesicles. References Further reading * * * * * * * * * {{Calcium-binding proteins S100 proteins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calretinin
Calretinin, also known as calbindin 2 (formerly 29 kDa calbindin), is a calcium-binding protein involved in calcium signaling. In humans, the calretinin protein is encoded by the ''CALB2'' gene. Function This gene encodes an intracellular calcium-binding protein belonging to the troponin C superfamily. Members of this protein family have six EF-hand domains which bind calcium. This protein plays a role in diverse cellular functions, including message targeting and intracellular calcium buffering. Calretinin is abundantly expressed in neurons including retina (which gave it the name) and cortical interneurons. Expression was found in different neurons than that of the similar vitamin D-dependent calcium-binding protein, calbindin-28kDa. Calretinin has an important role as a modulator of neuronal excitability including the induction of long-term potentiation. Loss of expression of calretinin in hippocampal interneurons has been suggested to be relevant in temporal lobe epil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TRPV6
TRPV6 is a membrane calcium (Ca2+) channel protein which is particularly involved in the first step in Ca2+absorption in the intestine. Classification Transient Receptor Potential Vanilloid subfamily member 6 (TRPV6) is an epithelial Ca2+ channel that belongs to the transient receptor potential family (TRP) of proteins. The TRP family is a group of channel proteins critical for ionic homeostasis and the perception of various physical and chemical stimuli. TRP channels can detect temperature, osmotic pressure, olfaction, taste, and mechanical forces. The human genome encodes for 28 TRP channels, which include six TRPV channels. The high Ca2+-selectivity of TRPV5 and TRPV6 makes these channels distinct from the other four TRPV channels (TRPV1-TRPV4). TRPV5 and TRPV6 are involved in Ca2+ transport, whereas TRPV1 through TRPV3 are heat sensors with different temperature threshold for activation, and TRPV4 is involved in sensing osmolarity. Genetic defects in TRPV6 gene are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium-binding Protein
Calcium-binding proteins are proteins that participate in calcium cell signaling pathways by binding to Ca2+, the calcium ion that plays an important role in many cellular processes. Calcium-binding proteins have specific domains that bind to calcium and are known to be heterogeneous. One of the functions of calcium binding proteins is to regulate the amount of free (unbound) Ca2+ in the cytosol of the cell. The cellular regulation of calcium is known as calcium homeostasis. Types Many different calcium-binding proteins exist, with different cellular and tissue distribution and involvement in specific functions. Calcium binding proteins also serve an important physiological role for cells. The most ubiquitous Ca2+-sensing protein, found in all eukaryotic organisms including yeasts, is calmodulin. Intracellular storage and release of Ca2+ from the sarcoplasmic reticulum is associated with the high-capacity, low-affinity calcium-binding protein calsequestrin. Calretinin is anoth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EF-hands
The EF hand is a helix–loop–helix structural domain or ''motif'' found in a large family of calcium-binding proteins. The EF-hand motif contains a helix–loop–helix topology, much like the spread thumb and forefinger of the human hand, in which the Ca2+ ions are coordinated by ligands within the loop. The motif takes its name from traditional nomenclature used in describing the protein parvalbumin, which contains three such motifs and is probably involved in muscle relaxation via its calcium-binding activity. The EF-hand consists of two alpha helices linked by a short loop region (usually about 12 amino acids) that usually binds calcium ions. EF-hands also appear in each structural domain of the signaling protein calmodulin and in the muscle protein troponin-C. Calcium ion binding site The calcium ion is coordinated in a pentagonal bipyramidal configuration. The six residues involved in the binding are in positions 1, 3, 5, 7, 9 and 12; these residues are den ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin D
Vitamin D is a group of structurally related, fat-soluble compounds responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, along with numerous other biological functions. In humans, the most important compounds within this group are vitamin D3 ( cholecalciferol) and vitamin D2 ( ergocalciferol). Unlike the other twelve vitamins, vitamin D is only conditionally essential, as with adequate skin exposure to the ultraviolet B (UVB) radiation component of sunlight there is synthesis of cholecalciferol in the lower layers of the skin's epidermis. For most people, skin synthesis contributes more than diet sources. Vitamin D can also be obtained through diet, food fortification and dietary supplements. In the U.S., cow's milk and plant-based milk substitutes are fortified with vitamin D3, as are many breakfast cereals. Government dietary recommendations typically assume that all of a person's vitamin D is taken by mouth, given the potential for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium-binding Proteins
Calcium-binding proteins are proteins that participate in calcium cell signaling pathways by binding to Ca2+, the calcium ion that plays an important role in many cellular processes. Calcium-binding proteins have specific domains that bind to calcium and are known to be heterogeneous. One of the functions of calcium binding proteins is to regulate the amount of free (unbound) Ca2+ in the cytosol of the cell. The cellular regulation of calcium is known as calcium homeostasis. Types Many different calcium-binding proteins exist, with different cellular and tissue distribution and involvement in specific functions. Calcium binding proteins also serve an important physiological role for cells. The most ubiquitous Ca2+-sensing protein, found in all eukaryotic organisms including yeasts, is calmodulin. Intracellular storage and release of Ca2+ from the sarcoplasmic reticulum is associated with the high-capacity, low-affinity calcium-binding protein calsequestrin. Calretinin is another t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EF Hand
The EF hand is a helix–loop–helix structural domain or ''motif'' found in a large family of calcium-binding proteins. The EF-hand motif contains a helix–loop–helix topology, much like the spread thumb and forefinger of the human hand, in which the Ca2+ ions are coordinated by ligands within the loop. The motif takes its name from traditional nomenclature used in describing the protein parvalbumin, which contains three such motifs and is probably involved in muscle relaxation via its calcium-binding activity. The EF-hand consists of two alpha helices linked by a short loop region (usually about 12 amino acids) that usually binds calcium ions. EF-hands also appear in each structural domain of the signaling protein calmodulin and in the muscle protein troponin-C. Calcium ion binding site The calcium ion is coordinated in a pentagonal bipyramidal configuration. The six residues involved in the binding are in positions 1, 3, 5, 7, 9 and 12; these residues are denoted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
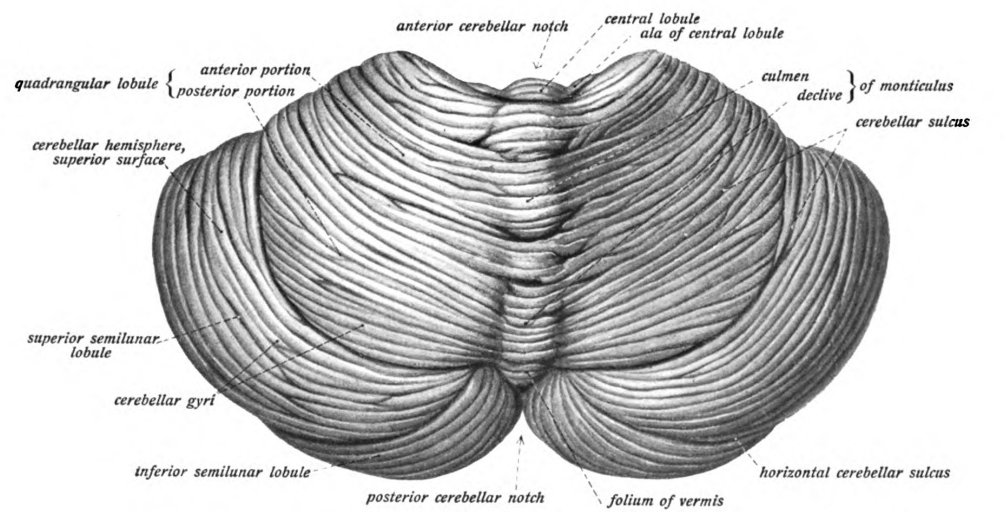
Cerebellum
The cerebellum (: cerebella or cerebellums; Latin for 'little brain') is a major feature of the hindbrain of all vertebrates. Although usually smaller than the cerebrum, in some animals such as the mormyrid fishes it may be as large as it or even larger. In humans, the cerebellum plays an important role in motor control and cognition, cognitive functions such as attention and language as well as emotion, emotional control such as regulating fear and pleasure responses, but its movement-related functions are the most solidly established. The human cerebellum does not initiate movement, but contributes to motor coordination, coordination, precision, and accurate timing: it receives input from sensory systems of the spinal cord and from other parts of the brain, and integrates these inputs to fine-tune motor activity. Cerebellar damage produces disorders in fine motor skill, fine movement, sense of balance, equilibrium, list of human positions, posture, and motor learning in humans. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's Peptide, polypeptide chain that is self-stabilizing and that Protein folding, folds independently from the rest. Each domain forms a compact folded Protein tertiary structure, three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or Disulfide bond, disulfide bridges. Domains often form functional units, such as the calcium-binding EF-hand, EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimera (protein), chimeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossils of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name comes from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceuticals for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |