|
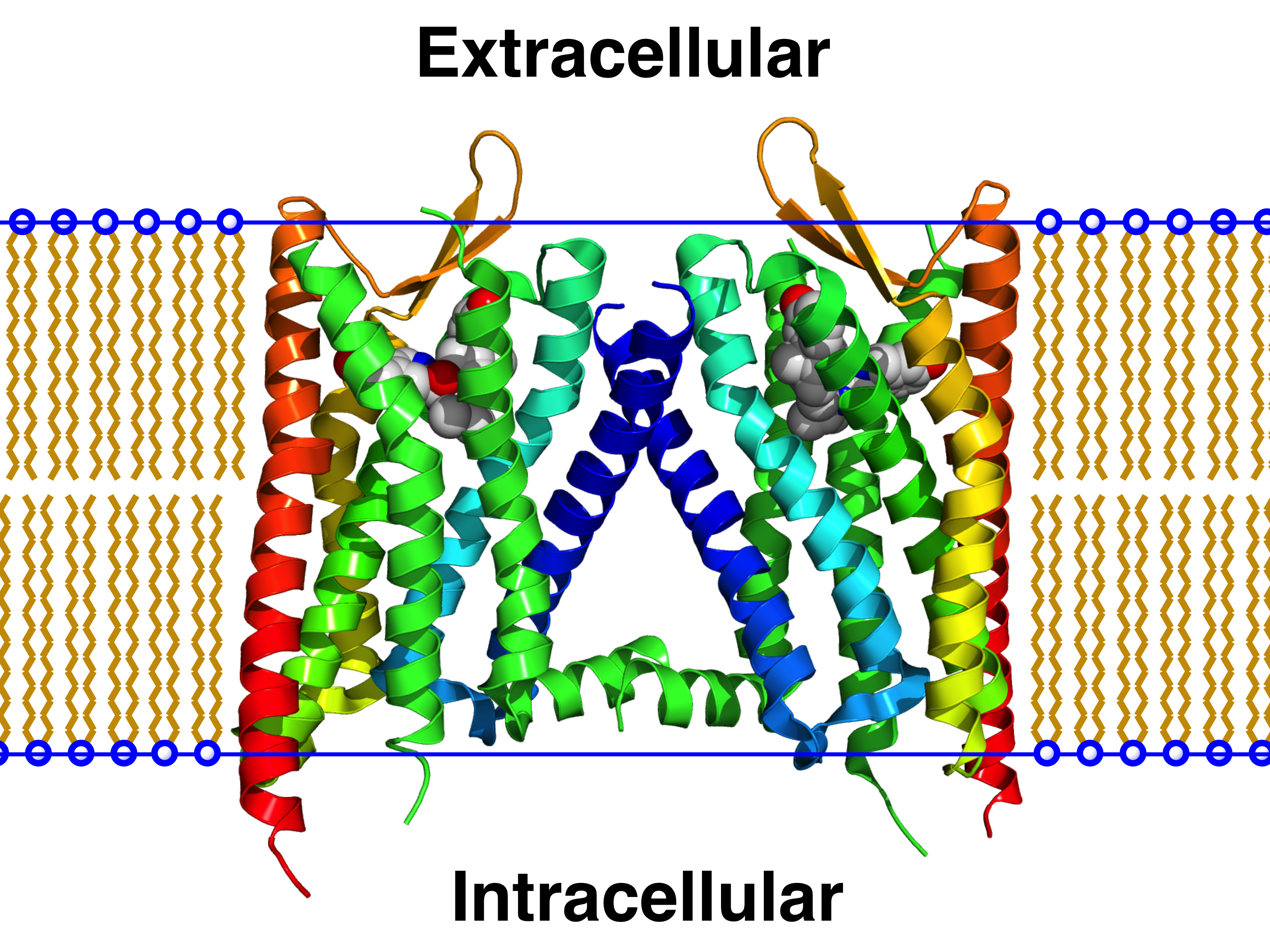
CB1 Receptor
Cannabinoid receptor 1 (CB1), is a G protein-coupled cannabinoid receptor that in humans is encoded by the ''CNR1'' gene. And discovered, by determination and characterization in 1988, and cloned in 1990 for the first time. The human CB1 receptor is expressed in the peripheral nervous system and central nervous system. It is activated by endogenous cannabinoids called endocannabinoids, a group of retrograde neurotransmitters that include lipids, such as anandamide and 2-arachidonoylglycerol; plant phytocannabinoids, such as docosatetraenoylethanolamide found in wild dagga, the compound tetrahydrocannabinol which is an active constituent of the psychoactive drug cannabis; and synthetic analogs of tetrahydrocannabinol. CB1 is antagonized by the phytocannabinoid tetrahydrocannabivarin at low doses and at higher doses, it activates the CB1 receptor as an agonist, but with less potency than tetrahydrocannabinol. The primary endogenous agonist of the human CB1 receptor is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein-coupled Receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops (three extracellular loops interacting with ligand molecules, three intracellular loops interacting with G proteins, an N-terminal extracellular region and a C-terminal intracellular region) of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Text was copied from this source, which is available under Attribution 2.5 Generic (CC BY 2.5) licence/ref> Ligands can bind either to the extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Cannabinoids
Synthetic cannabinoids, or neocannabinoids, are a class of designer drug molecules that bind to the same receptors to which cannabinoids ( THC, CBD and many others) in cannabis plants attach. These novel psychoactive substances should not be confused with synthetic phytocannabinoids (obtained by chemical synthesis) or synthetic endocannabinoids from which they are distinct in many aspects. Typically, synthetic cannabinoids are sprayed onto plant matter and are usually smoked, although they have also been ingested as a concentrated liquid form in the United States and United Kingdom since 2016. They have been marketed as herbal incense, or "herbal smoking blends", and sold under common names such as K2, spice, and synthetic marijuana. They are often labeled "not for human consumption" for liability defense. A large and complex variety of synthetic cannabinoids are designed in an attempt to avoid legal restrictions on cannabis, making synthetic cannabinoids designer drugs. Most s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allosteric Modulator
In pharmacology and biochemistry, allosteric modulators are a group of substances that bind to a receptor to change that receptor's response to stimuli. Some of them, like benzodiazepines or alcohol, function as psychoactive drugs. The site that an allosteric modulator binds to (i.e., an ''allosteric site'') is not the same one to which an endogenous agonist of the receptor would bind (i.e., an ''orthosteric site''). Modulators and agonists can both be called receptor ligands. Allosteric modulators can be 1 of 3 types either: positive, negative or neutral. Positive types increase the response of the receptor by increasing the probability that an agonist will bind to a receptor (i.e. affinity), increasing its ability to activate the receptor (i.e. efficacy), or both. Negative types decrease the agonist affinity and/or efficacy. Neutral types don't affect agonist activity but can stop other modulators from binding to an allosteric site. Some modulators also work as allosteric agonist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Îś-opioid Receptor
The μ-opioid receptors (MOR) are a class of opioid receptors with a high affinity for enkephalins and beta-endorphin, but a low affinity for dynorphins. They are also referred to as μ(''mu'')-opioid peptide (MOP) receptors. The prototypical μ-opioid receptor agonist is morphine, the primary psychoactive alkaloid in opium and for which the receptor was named, with mu being the first letter of Morpheus, the compound's namesake in the original Greek. It is an inhibitory G-protein coupled receptor that activates the Gi alpha subunit, inhibiting adenylate cyclase activity, lowering cAMP levels. Structure The structure of the inactive μ-opioid receptor has been determined with the antagonists β-FNA and alvimopan. Many structures of the active state are also available, with agonists including DAMGO, β-endorphin, fentanyl and morphine. The structure with the agonist BU72 has the highest resolution, but contains unexplained features that may be experimental artifac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypocretin (orexin) Receptor 1
Orexin receptor type 1 (Ox1R or OX1), also known as hypocretin receptor type 1 (HcrtR1), is a protein that in humans is encoded by the HCRTR1 gene. Function The orexin 1 receptor (OX1), is a G-protein coupled receptor that is heavily expressed in projections from the lateral hypothalamus and is involved in the regulation of feeding behaviour. OX1 selectively binds the orexin-A neuropeptide. It shares 64% identity with OX2. Ligands Agonists * Orexin-A Antagonists * RTIOX-276 - Selective OX1 antagonist * ACT-335827 - Selective OX1 antagonist * Almorexant - Dual OX1 and OX2 antagonist * Lemborexant - Dual OX1 and OX2 antagonist * Nemorexant - Dual OX1 and OX2 antagonist * SB-334,867 - Selective OX1 antagonist * SB-408,124 - Selective OX1 antagonist * SB-649,868 - Dual OX1 and OX2 antagonist * Suvorexant - Dual OX1 and OX2 antagonist An antagonist is a character in a story who is presented as the main enemy or rival of the protagonist and is often depicted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine Receptor D2
Dopamine receptor D2, also known as D2R, is a protein that, in humans, is encoded by the ''DRD2'' gene. After work from Paul Greengard's lab had suggested that dopamine receptors were the site of action of antipsychotic drugs, several groups, including those of Solomon H. Snyder and Philip Seeman used a radiolabeled antipsychotic drug to identify what is now known as the dopamine D2 receptor. The dopamine D2 receptor is the main receptor for most antipsychotic drugs. The structure of DRD2 in complex with the atypical antipsychotic risperidone has been determined. Function D2 receptors are coupled to Gi subtype of G protein. This G protein-coupled receptor inhibits adenylyl cyclase activity. In mice, regulation of D2R surface expression by the neuronal calcium sensor-1 (NCS-1) in the dentate gyrus is involved in exploration, synaptic plasticity and memory formation. Studies have shown potential roles for D2R in retrieval of fear memories in the prelimbic cortex and in d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adenosine A2A Receptor
The adenosine A2A receptor, also known as ADORA2A, is an adenosine receptor, and also denotes the human gene encoding it. Structure This protein is a member of the G protein-coupled receptor (GPCR) family which possess seven transmembrane alpha helices, as well as an extracellular N-terminus and an intracellular C-terminus. Furthermore, located in the intracellular side close to the membrane is a small alpha helix, often referred to as helix 8 (H8). The crystallographic structure of the adenosine A2A receptor reveals a ligand binding pocket distinct from that of other structurally determined GPCRs (i.e., the beta-2 adrenergic receptor and rhodopsin).; Below this primary ( orthosteric) binding pocket lies a secondary ( allosteric) binding pocket. The crystal-structure of A2A bound to the antagonist ZM241385 (PDB code: 4EIY) showed that a sodium-ion can be found in this location of the protein, thus giving it the name 'sodium-ion binding pocket'. Heteromers The actions of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein–coupled Receptor
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. They are coupled with G proteins. They pass through the cell membrane seven times in the form of six loops (three extracellular loops interacting with ligand molecules, three intracellular loops interacting with G proteins, an N-terminal extracellular region and a C-terminal intracellular region) of amino acid residues, which is why they are sometimes referred to as seven-transmembrane receptors. Text was copied from this source, which is available under Attribution 2.5 Generic (CC BY 2.5) licence/ref> Ligands can bind either to the extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GPCR Oligomer
A GPCR oligomer is a protein complex that consists of a small number ( ''oligoi'' "a few", ''méros'' "part, piece, component") of G protein-coupled receptors (GPCRs). It is held together by covalent bonds or by intermolecular forces. The subunits within this complex are called protomers, while unconnected receptors are called monomers. Receptor homomers consist of identical protomers, while heteromers consist of different protomers. Receptor homodimers – which consist of two identical GPCRs – are the simplest homomeric GPCR oligomers. Receptor heterodimers – which consist of two different GPCRs – are the simplest heteromeric GPCR oligomers. The existence of receptor oligomers is a general phenomenon, whose discovery has superseded the prevailing paradigmatic concept of the function of receptors as plain monomers, and has far-reaching implications for the understanding of neurobiological diseases as well as for the development of drugs. Discovery For a long time ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterodimer
In biochemistry, a protein dimer is a macromolecular complex or multimer formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins while a protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and hetero ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homodimer
In biochemistry, a protein dimer is a macromolecular complex or protein multimer, multimer formed by two protein monomers, or single proteins, which are usually Non-covalent interaction, non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", ''wikt:di-#Prefix, di-'' + ''wikt:-mer#Suffix, -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins while a protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein IKBKG, NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endogenous Agonist
In pharmacology, an endogenous agonist for a particular receptor is a compound naturally produced by the body which binds to and activates that receptor. For example, the primary endogenous agonist for serotonin receptors is serotonin, and the primary endogenous agonist for dopamine receptors is dopamine.Goodman and Gilman's Manual of Pharmacology and Therapeutics. (11th edition, 2008). p14. In general, receptors for small molecule neurotransmitters such as serotonin will have only one endogenous agonist, but often have many different receptor subtypes (e.g. 13 different receptors for serotonin). On the other hand, neuropeptide receptors tend to have fewer subtypes, but may have several different endogenous agonists. This allows for a high degree of complexity in the body's signalling system, with different tissues often showing quite distinct responses to a particular ligand. Some endogenous antagonists and inverse agonists are also known (e.g., kynurenic acid at the NMDA re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |