|
Benzopyrans
4H-1-Benzopyran is an organic compound with the formula . It is one of two isomers of benzopyran, the other being 2H-1-benzopyran, which is more prevalent. It can be viewed as the fusion of a benzene ring to a heterocyclic pyran ring. Some benzopyrans have shown anticancerous activity '' in vitro''. The radical form of benzopyran is paramagnetic. The unpaired electron is delocalized over the whole benzopyran molecule, rendering it less reactive than one would expect otherwise. A similar example is the cyclopentadienyl radical. Commonly, benzopyran is encountered in the reduced state, in which it is partially saturated with one hydrogen atom, introducing a tetrahedral CH2 group in the pyran ring. Therefore, there are many structural isomers owing to the multiple possible positions of the oxygen atom and the tetrahedral carbon atom: Nomenclature According to current IUPAC The International Union of Pure and Applied Chemistry (IUPAC ) is an international federatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzopyrone
Benzopyrone may refer to either of two ketone derivatives of benzopyran 4H-1-Benzopyran is an organic compound with the formula . It is one of two isomers of benzopyran, the other being 2H-1-Benzopyran, 2H-1-benzopyran, which is more prevalent. It can be viewed as the fusion of a benzene ring to a heterocyclic pyra ... which constitute the core skeleton of many flavonoid compounds: * Chromone (1-benzopyran-4-one) * Coumarin (1-benzopyran-2-one) Certain simple benzopyrones have clinical medical value as an edema modifiers. Coumarin and other benzopyrones, such as 5,6 benzopyrone, 1,2 benzopyrone, diosmin and others are known to stimulate macrophages to degrade extracellular albumin, allowing faster resorption of edematous fluids. Naturally occurring coumarin is also the basis for various 4-hydroxybenzopyrone-based molecules which occur naturally dicoumarol and are made synthetically warfarin and function as anticoagulants. References {{reflist Benzopyrans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2H-1-Benzopyran
2H-1-Benzopyran is an organic compound with the formula . It is one of two isomers of benzopyran, the other being 4H-1-Benzopyran, 4H-1-benzopyran, which is less prevalent. It can be viewed as the fusion of a benzene ring to a heterocyclic pyran ring. Some benzopyrans have shown anticancerous activity ''in vitro''. The radical (chemistry), radical form of benzopyran is paramagnetic. The unpaired electron is delocalized over the whole benzopyran molecule, rendering it less reactive than one would expect otherwise. A similar example is the cyclopentadienyl radical. Commonly, benzopyran is encountered in the reduced state, in which it is partially saturated with one hydrogen atom, introducing a tetrahedral CH2 group in the pyran ring. Therefore, there are many structural isomers owing to the multiple possible positions of the oxygen atom and the tetrahedral carbon atom: Nomenclature According to current IUPAC nomenclature, the name chromene used in previous recommendations is r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
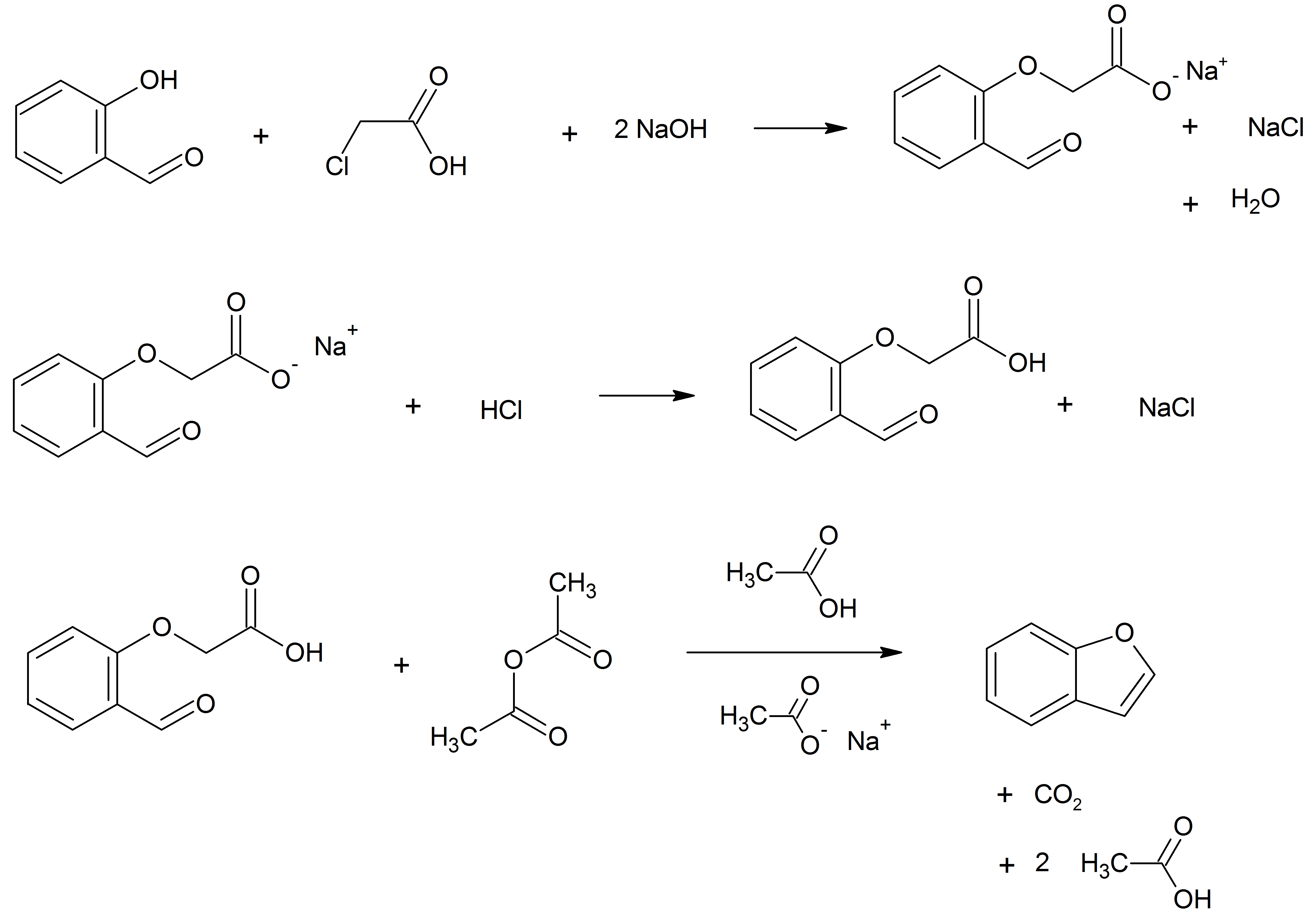
Benzofuran
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethyl phenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. * Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : * Cycloisomerization of alkyne ortho-substituted phenols: : Related compounds * Substituted benzofurans * Dibenzofuran, an analog w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation, ppm by mass. As an Aromaticity, aromatic hydrocarbon, naphthalene's structure consists of a fused pair of benzene rings. It is the main ingredient of traditional mothballs. History In the early 1820s, two separate reports described a white solid with a pungent odor derived from the distillation of coal tar. In 1821, John Kidd (chemist), John Kidd cited these two disclosures and then described many of this substance's properties and the means of its production. He proposed the name ''naphthaline'', as it had been derived from a kind of naphtha (a broad term encompassing any volatile, flammable liquid hydrocarbon mixture, including coal tar). Naphthalene's chemical formula was determined by Michael Faraday in 1826. The structure of two f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Preferred IUPAC Name
In chemical nomenclature, a preferred IUPAC name (PIN) is a unique name, assigned to a chemical substance and preferred among all possible names generated by IUPAC nomenclature. The "preferred IUPAC nomenclature" provides a set of rules for choosing between multiple possibilities in situations where it is important to decide on a unique name. It is intended for use in legal and regulatory situations. Preferred IUPAC names are applicable only for organic compounds, to which the IUPAC (International Union of Pure and Applied Chemistry) has the definition as compounds which contain at least a single carbon atom but no alkali, alkaline earth or transition metals and can be named by the nomenclature of organic compounds (see below). Rules for the remaining organic and inorganic compounds are still under development. The concept of PINs is defined in the introductory chapter and chapter 5 of the ''"Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013"'' ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national List of chemistry societies, chemistry societies, national Academy of Sciences, academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl
Cyclopentadienyl can refer to * Cyclopentadienyl anion, or cyclopentadienide, ** Cyclopentadienyl ligand * Cyclopentadienyl radical, • * Cyclopentadienyl cation, See also * Pentadienyl {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |