|
Benzofuran
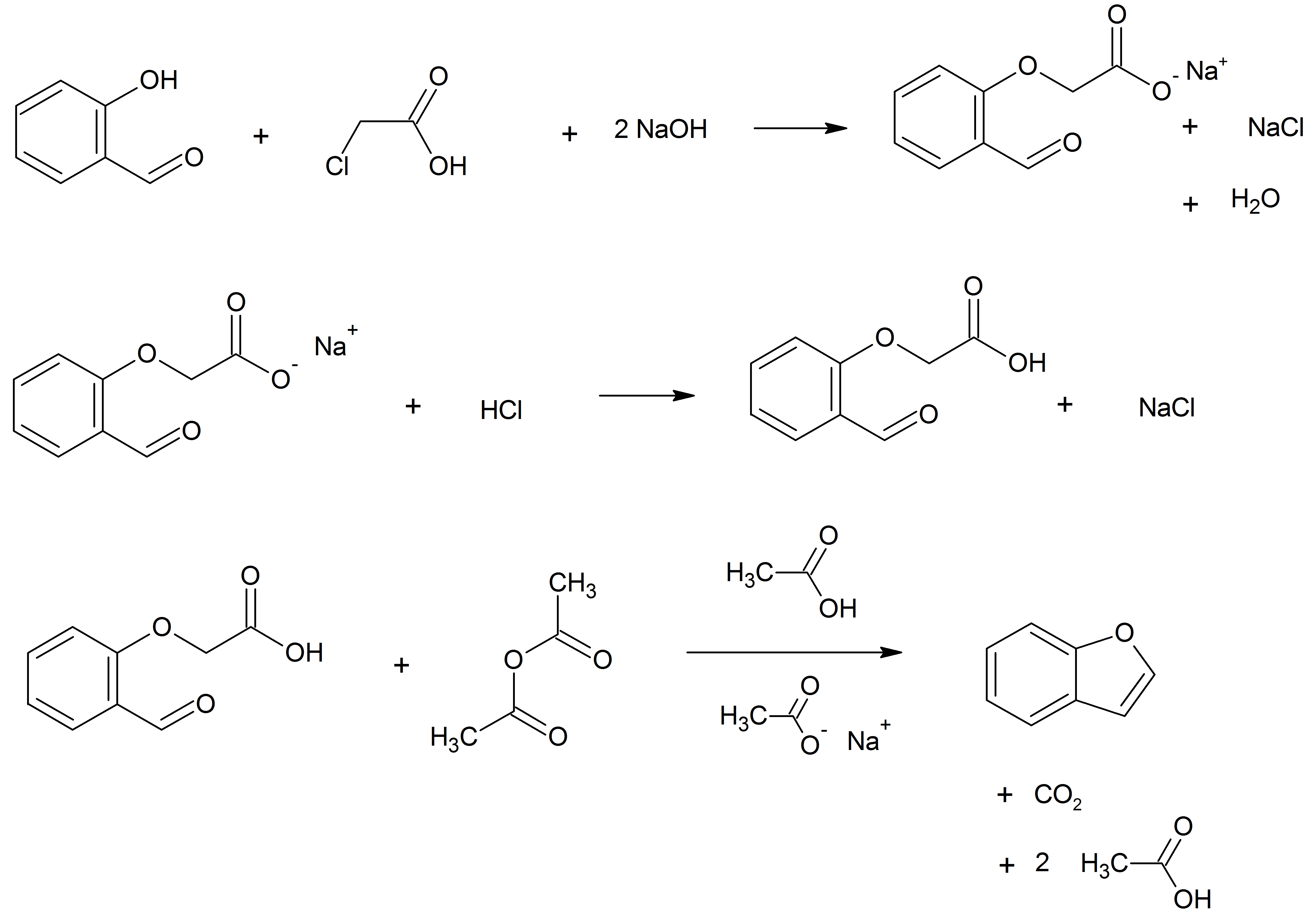
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethyl phenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. * Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : * Cycloisomerization of alkyne ortho-substituted phenols: : Related compounds * Substituted benzofurans * Dibenzofuran, an analog w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Cumaranone
2-Coumaranone (sometimes also called 2-Cumaranone) is a bicyclic heteroaromatic compound in which a six-membered benzene ring is Annulation, annulated with a five-membered γ-butyrolactone ring. The 2(3''H'')-benzofuranone can also be considered as a lactone of (2-hydroxyphenyl)acetic acid. The benzofuranone basic structure is the basis of some natural products – such as rosmadial, which is isolatable from Rosemary Oil, rosemary oil, and some substances with high pharmacological activity, such as griseofulvin and rifampicin. Furthermore, 2-cumaranone is utilized as a starting material for the preparation of chemiluminescent and fluorescent dyes, for synthetic pharmaceutical agents, like the antiarrhythmic drug dronedarone, and especially for the fungicide azoxystrobin. Occurrence and synthesis In 1884, Adolf von Baeyer and Paul Fritsch (chemist), Paul Fritsch disclosed the synthesis of 2-coumaranone, which they described as the lactone of ''o''-oxyphenylacetic acid, through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzofurans Via Cycloisomerization Of Alkynes
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration reaction, dehydration (cyclication) of the resulting ether and decarboxylation. *Perkin rearrangement, where a coumarin is reacted with a hydroxide: : *Diels–Alder reaction of Nitroalkene, nitro vinyl furans with various dienophiles: : *Isomerization, Cycloisomerization of alkyne Arene substitution pattern, ortho-substituted phenols: : Related compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzofuran
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants. Production Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethyl phenol. Laboratory methods Benzofurans can be prepared by various methods in the laboratory. Notable examples include: *''O''-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation. * Perkin rearrangement, where a coumarin is reacted with a hydroxide: : * Diels–Alder reaction of nitro vinyl furans with various dienophiles: : * Cycloisomerization of alkyne ortho-substituted phenols: : Related compounds * Substituted benzofurans * Dibenzofuran, an analog w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salicylaldehyde
Salicylic aldehyde (2-hydroxybenzaldehyde) is an organic compound with the formula . Along with 3-hydroxybenzaldehyde and 4-hydroxybenzaldehyde, it is one of the three isomers of hydroxybenzaldehyde. This colorless oily liquid has a bitter almond odor at higher concentration. Salicylaldehyde is a precursor to coumarin and a variety of chelating agents. Production Salicylaldehyde is produced by condensation of phenol with formaldehyde to give hydroxybenzyl alcohol, which is oxidized to the aldehyde. Salicylaldehydes in general are prepared by ortho-selective formylation reactions from the corresponding phenol, for instance by the Duff reaction, Reimer–Tiemann reaction, or by treatment with paraformaldehyde in the presence of magnesium chloride and a base. : Natural occurrences Salicylaldehyde is a characteristic aroma component of buckwheat. Salicylaldehyde also occurs in the larval defensive secretions of several leaf beetle species that belong the subtribe Chryso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compound
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of py ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perkin Rearrangement
The Perkin rearrangement (coumarin–benzofuran ring contraction) is a rearrangement reaction in which a 2-halocoumarin in the presence of hydroxide undergoes a ring contraction to form a benzofuran. The name reaction recognizes William Henry Perkin, who first reported it in 1870. Several proposals have been made for the reaction mechanism, all of which involve initial opening of the lactone to give a carboxylate and phenolate Phenolates (also called phenoxides) are anions, salt (chemistry), salts, and esters of phenols, containing the phenolate ion. They may be formed by reaction of phenols with strong base. Properties Alkali metal phenolates, such as sodium phenoxi .... : References {{organic-chem-stub Benzofurans Coumarins Name reactions Rearrangement reactions Ring contraction reactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indole
Indole is an organic compound with the formula . Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. It has been identified in cannabis. It is the main volatile compound in stinky tofu. When indole is a substituent on a larger molecule, it is called an ''indolyl group'' by systematic nomenclature. Indole undergoes electrophilic substitution, mainly at position 3 (see diagram in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furans
Furan is a heterocyclic organic compound, consisting of a five-membered aromatic ring with four carbon atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly volatile liquid with a boiling point close to room temperature. It is soluble in common organic solvents, including alcohol, ether, and acetone, and is slightly soluble in water. Its odor is "strong, ethereal; chloroform-like". It is toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals. History The name "furan" comes from the Latin ''furfur'', which means bran (furfural is produced from bran). The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 and characterised nine years later by John Stenhouse. Furan itself was first prepared by Heinrich Limpr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Furan
Furan is a Heterocyclic compound, heterocyclic organic compound, consisting of a five-membered aromatic Ring (chemistry), ring with four carbon Atom, atoms and one oxygen atom. Chemical compounds containing such rings are also referred to as furans. Furan is a colorless, flammable, highly Volatility (chemistry), volatile liquid with a boiling point close to room temperature. It is soluble in common organic Solvent, solvents, including ethanol, alcohol, diethyl ether, ether, and acetone, and is slightly soluble in water. Its odor is "strong, ethereal; chloroform-like". It is toxic and may be carcinogenic in humans. Furan is used as a starting point for other speciality chemicals. History The name "furan" comes from the Latin ''furfur'', which means bran (furfural is produced from bran). The first furan derivative to be described was 2-furoic acid, by Carl Wilhelm Scheele in 1780. Another important derivative, furfural, was reported by Johann Wolfgang Döbereiner in 1831 an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coal Tar
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It is a type of creosote. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasis and seborrheic dermatitis ( dandruff). It may be used in combination with ultraviolet light therapy. Industrially it is a railroad tie preservative and used in the surfacing of roads. Coal tar was listed as a known human carcinogen in the first Report on Carcinogens from the U.S. Federal Government, issued in 1980. Coal tar was discovered circa 1665 and used for medical purposes as early as the 1800s. Circa 1850, the discovery that it could be used as the main raw material for the synthesis of dyes engendered an entire industry. It is on the World Health Organization's List of Essential Medicines. Coal tar is available as a generic medication and over the counter. Side effects include skin irritation, sun sensitivity, aller ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psoralen
Psoralen (also called psoralene) is the parent compound in a family of naturally occurring organic compounds known as the linear furanocoumarins. It is structurally related to coumarin by the addition of a fused furan ring, and may be considered as a derivative of umbelliferone. Psoralen occurs naturally in the seeds of ''Psoralea corylifolia'', as well as in the common fig, celery, parsley, Zanthoxylum, West Indian satinwood, and in all citrus fruits. It is widely used in PUVA (psoralen + Ultraviolet#Subtypes, UVA) treatment for psoriasis, eczema, vitiligo, and cutaneous T-cell lymphoma; these applications are typically through the use of medications such as Methoxsalen. Many furanocoumarins are extremely toxic to fish, and some are deposited in streams in Indonesia to catch fish. Uses Psoralen is a mutagen, and is used for this purpose in molecular biology research. Psoralen Intercalation (biochemistry), intercalates into DNA and on exposure to ultraviolet (UVA) radiation can f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |