|
Butyramide
Butyramide is the amide of butyric acid. It has the molecular formula C3H7CONH2. It is a white solid that is freely soluble in water and ethanol, but slightly soluble in diethyl ether. At room temperature, butyramide is a crystalline solid and in contrast to butyric acid, it is devoid of an unpleasant, rancid smell. Synthesis Butyramide can be synthesized by: * catalytic hydration of butyronitrile; * reaction of butyryl chloride with ammonium salts; * reduction of butyraldoxime. Derivatives Some of its derivatives have shown preliminary strong anticonvulsive activity and inhibitory action on histone In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn a ... deacetylases, which are crucial enzymes controlling the proliferative or differentiation status of most cells. See also * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyric Acid
Butyric acid (; from grc, βούτῡρον, meaning "butter"), also known under the systematic name butanoic acid, is a straight-chain alkyl carboxylic acid with the chemical formula CH3CH2CH2CO2H. It is an oily, colorless liquid with an unpleasant odor. Isobutyric acid (2-methylpropanoic acid) is an isomer. Salts and esters of butyric acid are known as butyrates or butanoates. The acid does not occur widely in nature, but its esters are widespread. It is a common industrial chemical and an important component in the mammalian gut. History Butyric acid was first observed in impure form in 1814 by the French chemist Michel Eugène Chevreul. By 1818, he had purified it sufficiently to characterize it. However, Chevreul did not publish his early research on butyric acid; instead, he deposited his findings in manuscript form with the secretary of the Academy of Sciences in Paris, France. Henri Braconnot, a French chemist, was also researching the composition of butter and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isobutyramide
Isobutyramide in chemistry is an amide with the molecular formula C4H9NO. Isobutyramide can also refer to the functional group with the following chemical formula: R-NH-CO-CH(CH3)2. : See also * Butyramide Butyramide is the amide of butyric acid. It has the molecular formula C3H7CONH2. It is a white solid that is freely soluble in water and ethanol, but slightly soluble in diethyl ether. At room temperature, butyramide is a crystalline solid and i ... Carboxamides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Merck Index
''The Merck Index'' is an encyclopedia of chemicals, drugs and biologicals with over 10,000 monograph on single substances or groups of related compounds published online by the Royal Society of Chemistry. History The first edition of the Merck's Index was published in 1889 by the German chemical company Emanuel Merck and was primarily used as a sales catalog for Merck's growing list of chemicals it sold. The American subsidiary was established two years later and continued to publish it. During World War I the US government seized Merck's US operations and made it a separate American "Merck" company that continued to publish the Merck Index. In 2012 the Merck Index was licensed to the Royal Society of Chemistry. An online version of The Merck Index, including historic records and new updates not in the print edition, is commonly available through research libraries. It also includes an appendix with monographs on organic named reactions. The 15th edition was published in A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of sugars by yeasts or via petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the synthesis of organic compounds, and as a fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world production of ethanol was , coming mostly from Brazil and the U.S. Etymology ''Ethanol'' is the systematic name defined by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyronitrile
Butyronitrile or butanenitrile or propyl cyanide, is a nitrile with the formula C3H7CN. This colorless liquid is miscible with most polar organic solvents. Uses Butyronitrile is mainly used as a precursor to the poultry drug amprolium.Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" ''Ullmann's Encyclopedia of Industrial Chemistry'' 2002, Wiley-VCH, Weinheim. It also has recognized use in the synthesis of Etifelmine. Synthesis Butyronitrile is prepared industrially by the ammoxidation of ''n''-butanol: :C3H7CH2OH + NH3 + O2 → C3H7CN + 3 H2O Occurrence in space Butyronitrile has been detected in the Large Molecule Heimat The Large Molecule Heimat is a dense gas cloud located in the molecular cloud Sagittarius B2. Many species of molecule, including aminoacetonitrile (a molecule related to glycine), ethyl formate, and butyronitrile Butyronitrile or butanenitril .... References External links NIST Chemistry WebBook page f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butyryl Chloride
Butyryl chloride is an organic compound with the chemical formula CH3CH2CH2C(O)Cl. It is a colorless liquid with a pungent odor. Butyryl chloride is soluble in aprotic organic solvents, but it reacts readily with water and alcohols. It is usually produced by chlorination of butyric acid. Reactions Like related acyl chlorides, butyryl chloride hydrolyzes readily: :CH3CH2CH2C(O)Cl + H2O → CH3CH2CH2CO2H + HCl Alcohols react to give esters: :CH3CH2CH2C(O)Cl + ROH → CH3CH2CH2CO2R + HCl Amines react to give amides: :CH3CH2CH2C(O)Cl + R2NH → CH3CH2CH2C(O)NR2 + HCl Derivatives of butyryl chloride are used in manufacturing pesticides, pharmaceuticals, perfume fixative, polymerization catalyst, and dyestuffs. Butyryl chloride is also commonly used as an intermediate for organic synthesis for the preparation of pharmaceuticals, agrochemicals, dyes, esters, and peroxide compounds.http://chemicalland21.com/specialtychem/perchem/N-BUTYRYL%20CHLORIDE.htm Safety Butyr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by organic groups (indicated by R). Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors): :H+ + NH3 -> H4 The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule: : H4 + B- -> HB + NH3 Thus, treatment of concentrated solutions of ammonium salts with strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions: :H2O + NH3 OH- + H4 The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Redox Reaction
Organic reductions or organic oxidations or organic redox reactions are redox reactions that take place with organic compounds. In organic chemistry oxidations and reductions are different from ordinary redox reactions, because many reactions carry the name but do not actually involve electron transfer.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Instead the relevant criterion for organic oxidation is gain of oxygen and/or loss of hydrogen, respectively.''Organic Redox Systems: Synthesis, Properties, and Applications'', Tohru Nishinaga 2016 Simple functional groups can be arranged in order of increasing oxidation state. The oxidation numbers are only an approximation: When methane is oxidized to carbon dioxide its oxidation number changes from −4 to +4. Classical reductions include alkene reduction to alkanes and classical oxidations include oxidation of alcohols to aldehydes. In oxidati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticonvulsive
Anticonvulsants (also known as antiepileptic drugs or recently as antiseizure drugs) are a diverse group of pharmacological agents used in the treatment of epileptic seizures. Anticonvulsants are also increasingly being used in the treatment of bipolar disorder and borderline personality disorder, since many seem to act as mood stabilizers, and for the treatment of neuropathic pain. Anticonvulsants suppress the excessive rapid firing of neurons during seizures. Anticonvulsants also prevent the spread of the seizure within the brain. Conventional antiepileptic drugs may block sodium channels or enhance γ-aminobutyric acid ( GABA) function. Several antiepileptic drugs have multiple or uncertain mechanisms of action. Next to the voltage-gated sodium channels and components of the GABA system, their targets include GABAA receptors, the GAT-1 GABA transporter, and GABA transaminase. Additional targets include voltage-gated calcium channels, SV2A, and α2δ. By blocking sodium or cal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
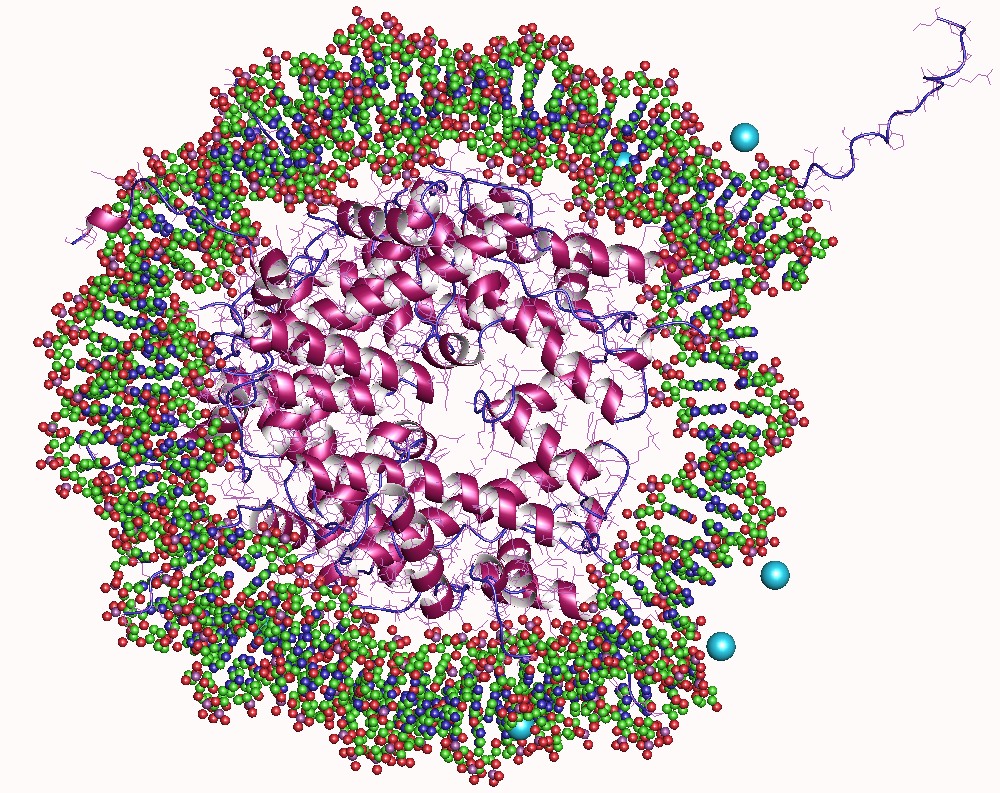
Histone
In biology, histones are highly basic proteins abundant in lysine and arginine residues that are found in eukaryotic cell nuclei. They act as spools around which DNA winds to create structural units called nucleosomes. Nucleosomes in turn are wrapped into 30- nanometer fibers that form tightly packed chromatin. Histones prevent DNA from becoming tangled and protect it from DNA damage. In addition, histones play important roles in gene regulation and DNA replication. Without histones, unwound DNA in chromosomes would be very long. For example, each human cell has about 1.8 meters of DNA if completely stretched out; however, when wound about histones, this length is reduced to about 90 micrometers (0.09 mm) of 30 nm diameter chromatin fibers. There are five families of histones which are designated H1/H5 (linker histones), H2, H3, and H4 (core histones). The nucleosome core is formed of two H2A-H2B dimers and a H3-H4 tetramer. The tight wrapping of DNA around h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |