|
Berry Mechanism
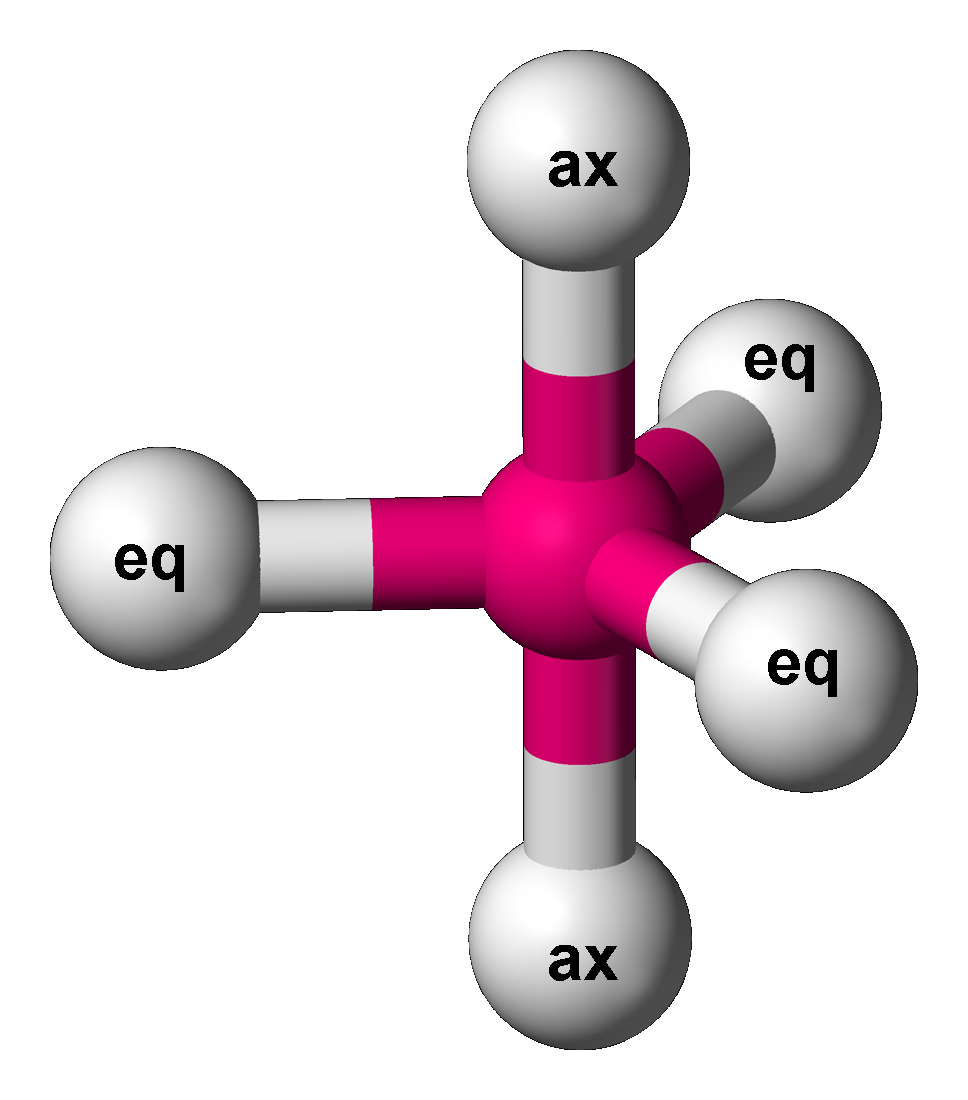
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerization, isomerize by exchanging the two axial ligands (see the figure) for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation and most commonly occurs in trigonal bipyramidal molecular geometry, trigonal bipyramidal molecules such as PF5, PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry, who first described this mechanism in 1960.R. S. Berry, 1960"Correlation of rates of intramolecular tunneling processes, with application to some Group V compounds" ''J. Chem. Phys.'' 32:933–938, ; accessed 28 May 2014.M. Cass, Mimi Hii, K. K. Hii, H. S. Rzepa, 2005"Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine Pentafluoride
Iodine pentafluoride is an interhalogen compound with chemical formula IF5. It is one of the fluorides of iodine. It is a colorless liquid, although impure samples appear yellow. It is used as a fluorination reagent and even a solvent in specialized syntheses. Preparation It was first synthesized by Henri Moissan in 1891 by burning solid iodine in fluorine gas. This exothermic reaction is still used to produce iodine pentafluoride, although the reaction conditions have been improved. :I2 + 5 F2 → 2 IF5 Reactions IF5 reacts vigorously with water forming hydrofluoric acid and iodic acid: :IF5 + 3 H2O → HIO3 + 5 HF Upon treatment with fluorine, it converts to iodine heptafluoride: :IF5 + F2 → IF7 It has been used as a solvent for handling metal fluorides. For example, the reduction of osmium hexafluoride to osmium pentafluoride with iodine is conducted in a solution in iodine pentafluoride: :10 OsF6 + I2 → 10 OsF5 + 2 IF5 Primary amines react with iodi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Geometry
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths, bond angles, torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity, polarity, phase of matter, color, magnetism and biological activity. The angles between bonds that an atom forms depend only weakly on the rest of a molecule, i.e. they can be understood as approximately local and hence transferable properties. Determination The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR, microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques. X-ray crystallography, neutron diffraction and electron diffraction can g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluxional Molecule
In chemistry and molecular physics, fluxional (or non-rigid) molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening (beyond that dictated by the Heisenberg uncertainty principle) due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling and other methods. Spectroscopic studies Many organometallic compounds exhibit fluxionality. Fluxionality is, however, pervasive. NMR spectroscopy Temperature dependent changes in the NMR spectra result from dynamics associated with the fluxional molecules when those dynamics proceed at rates compar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ray–Dutt Twist
The Ray–Dutt twist is a mechanism proposed for the racemization of octahedral complexes containing three bidentate chelate rings. Such complexes typically adopt an octahedral molecular geometry in their ground states, in which case they possess helical chirality. The pathway entails formation of an intermediate of C2v point group symmetry. An alternative pathway that also does not break any metal-ligand bonds is called the Bailar twist. Both of these mechanism product complexes wherein the ligating atoms (X in the scheme) are arranged in an approximate trigonal prism. This pathway is called the Ray–Dutt twist in honor of Priyadaranjan Ray (not Prafulla Chandra Ray) and N. K. Dutt, inorganic chemists at the Indian Association for the Cultivation of Science abbr. ''IACS'' who proposed this process. See also * Pseudorotation * Bailar twist * Bartell mechanism * Berry mechanism * Fluxional molecule In chemistry and molecular physics, fluxional (or non-rigid) mole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bartell Mechanism
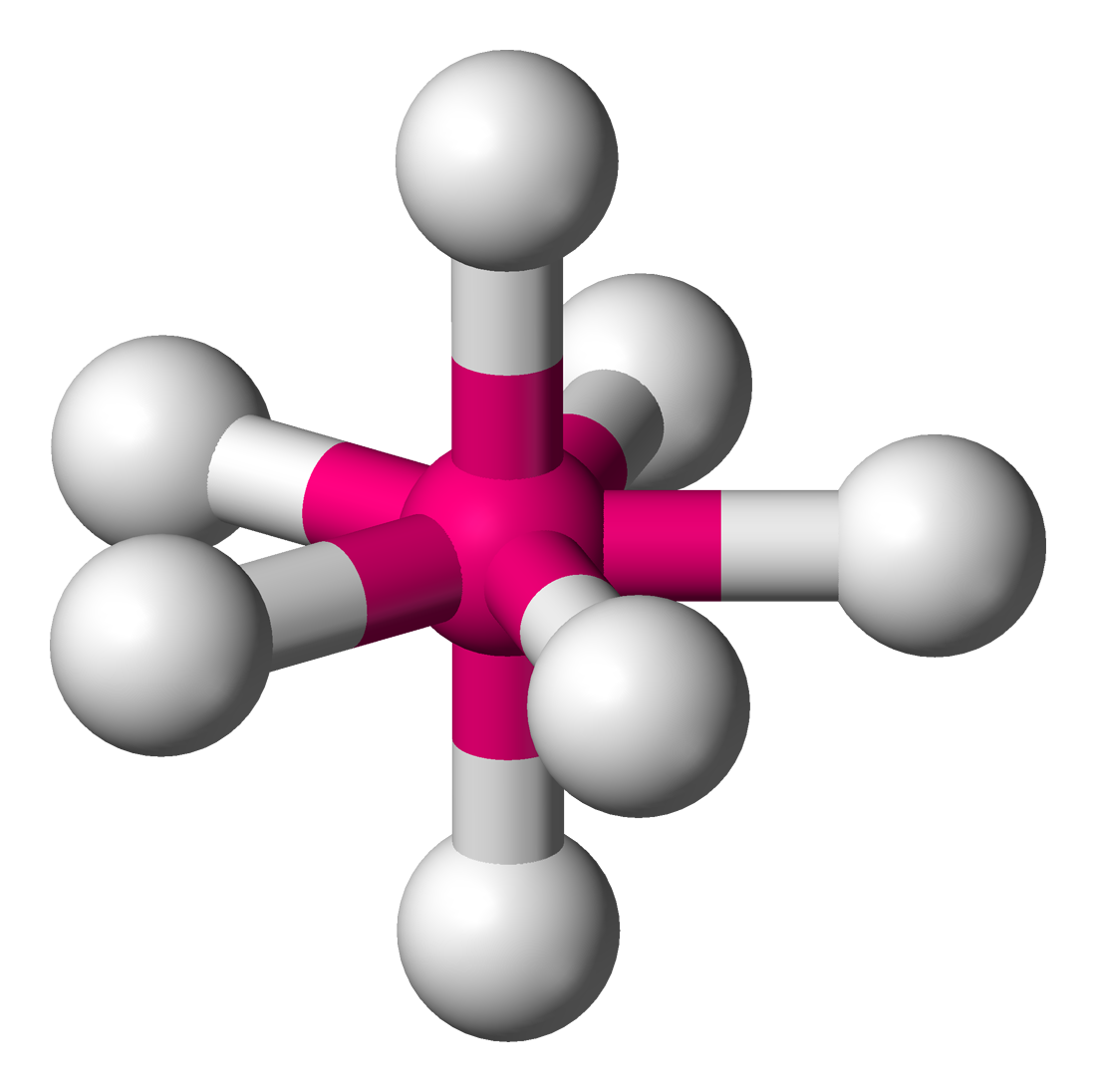
The Bartell mechanism is a pseudorotational mechanism similar to the Berry mechanism. It occurs only in molecules with a pentagonal bipyramidal molecular geometry, such as IF7. This mechanism was first predicted by H. B. Bartell. The mechanism exchanges the axial atoms with one pair of the equatorial atoms with an energy requirement of about 2.7 kcal/mol. Similarly to the Berry mechanism in square planar molecules, the symmetry of the intermediary phase of the vibrational mode is "chimeric"LS Bartell, MJ Rothman & A Gavezzotti, 1982, ''J. Chem. Phys.'' 76:4136-4413.M Cass, KK Hii & HS Rzepa, 2005, "Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition state normal vibrational modes", ''J. Chem. Educ.'' (online), 2005; se, accessed 28 May 2014 of other mechanisms; it displays characteristics of the Berry mechanism, a "lever" mechanism seen in pseud ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bailar Twist
Bailar ("dance" in Spanish) may refer to: People * Barbara A. Bailar (born 1935), American statistician * Benjamin F. Bailar (1934-2017), US Postmaster General from 1975 to 1978 * Gregor Bailar (born 1963), American technology executive * John C. Bailar Jr. (1904-1991), American chemist and professor * John C. Bailar III (1932–2016), American statistician * Schuyler Bailar (born 1996), American swimmer Other uses * "Bailar" (song), by DJ Deorro * Bailar twist, a chemical mechanism * Bailar, Iran, a village in Qazvin Province See also * Baila (other) {{disambiguation, surname, geo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudorotation
In chemistry, a pseudorotation is a set of intramolecular movements of attached groups (i.e., ligands) on a highly symmetric molecule, leading to a molecule indistinguishable from the initial one. The International Union of Pure and Applied Chemistry (IUPAC) defines a pseudorotation as a " stereoisomerization resulting in a structure that ''appears'' to have been produced by rotation of the entire initial molecule", the result of which is a "product" that is "superposable on the initial one, unless different positions are distinguished by substitution, including isotopic substitution." Well-known examples are the intramolecular isomerization of trigonal bipyramidal compounds by the Berry pseudorotation mechanism, and the out-of-plane motions of carbon atoms exhibited by cyclopentane, leading to the interconversions it experiences between its many possible conformers (envelope, twist). Note, no angular momentum is generated by this motion. In these and related examples, a smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berry Pseudorotation
The Berry mechanism, or Berry pseudorotation mechanism, is a type of vibration causing molecules of certain geometries to isomerize by exchanging the two axial ligands (see the figure) for two of the equatorial ones. It is the most widely accepted mechanism for pseudorotation and most commonly occurs in trigonal bipyramidal molecules such as PF5, though it can also occur in molecules with a square pyramidal geometry. The Berry mechanism is named after R. Stephen Berry, who first described this mechanism in 1960.R. S. Berry, 1960"Correlation of rates of intramolecular tunneling processes, with application to some Group V compounds" ''J. Chem. Phys.'' 32:933–938, ; accessed 28 May 2014.M. Cass, K. K. Hii, H. S. Rzepa, 2005"Mechanisms that interchange axial and equatorial atoms in fluxional processes: Illustration of the Berry pseudorotation, the turnstile and the lever mechanisms via animation of transition state normal vibrational modes" ''J. Chem. Educ.'' (online), 2005 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomerization. When the activation energy for the isomerization reaction is sufficiently small, both isomers can often be observed and the equilibrium ratio will shift in a temperature-dependent equilibrium with each other. Many values of the standard free energy difference, \Delta G^\circ, have been calculated, with good agreement between observed and calculated data. Examples and applications Alkanes Skeletal isomerization occurs in the cracking process, used in the petrochemical industry to convert straight chain alkanes to isoparaffins as exemplified in the conversion of normal octane to 2,5-dimethylhexane (an "isoparaffin"): : Fuels containing branched hydrocarbons are favored for internal combustion engines for their higher octan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Square Pyramidal Molecular Geometry
Square pyramidal geometry describes the shape of certain chemical compounds with the formula where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a Square pyramid, pyramid with a square base. The point group symmetry involved is of type C4v. The geometry is common for certain main group compounds that have a Stereochemistry, stereochemically-active lone pair, as described by VSEPR theory. Certain compounds crystallize in both the trigonal bipyramidal and the square pyramidal structures, notably . As a transition state in Berry pseudorotation As a trigonal bipyramidal molecule undergoes Berry pseudorotation, it proceeds via an intermediary stage with the square pyramidal geometry. Thus even though the geometry is rarely seen as the ground state, it is accessed by a low energy distortion from a trigonal bipyramid. Pseudorotation also occurs in square pyramidal molecules. Molecules with this geometry, as opposed to trigonal bipyramidal, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |