|
Basketane
Basketane is a polycyclic alkane with the chemical formula C10H12. The name is taken from its structural similarity to a basket shape. Basketane was first synthesized in 1966, independently by Masamune and Dauben and Whalen. Nomenclature Some compounds are named for objects seen in everyday life. Cubane, housane, and basketane were named accordingly. Synthesis One synthesis of basketane begins with a Diels–Alder reaction between cyclooctatetraene (1) and maleic anhydride (2), giving the polycyclic anhydride 3, which photoisomerizes in acetone via an intramolecular cyclization to give 4 at a 40% yield. Hydrolysis of the anhydride followed by treatment with lead tetraacetate affords the unsaturated basketene (5), which is then hydrogenated to basketane (6). : An alternative synthetic route with better overall yield uses 1,4-benzoquinone and cyclohexa-1,3-diene as starting materials. 1,4-Benzoquinone (1) is first converted to 2,5-dibromo-1,4-benzoquinone (2), which rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polycyclic Compound
In the field of organic chemistry, a polycyclic compound is an organic compound featuring several closed ring (chemistry), rings of atoms, primarily carbon. These ring substructures include cycloalkanes, aromaticity, aromatics, and other ring types. They come in sizes of three atoms and upward, and in combinations of linkages that include tethering (such as in biaryls), fusing (edge-to-edge, such as in anthracene and steroids), links via a single atom (such as in spiro compounds), bridged compounds, and longifolene. Though poly- literally means "many", there is some latitude in determining how many rings are required to be considered polycyclic; many smaller rings are described by specific prefixes (e.g., bicyclic molecule, bicyclic, tricyclic, tetracyclic, etc.), and so while it can refer to these, the title term is used with most specificity when these alternative names and prefixes are unavailable. In general, the term polycyclic includes polycyclic aromatic compounds, inclu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hunsdiecker Reaction
The Hunsdiecker reaction (also called the Borodin reaction or the Hunsdiecker–Borodin reaction) is a name reaction in organic chemistry whereby silver salts of carboxylic acids react with a halogen to produce an organic halide. It is an example of both a decarboxylation and a halogenation reaction as the product has one fewer carbon atoms than the starting material (lost as carbon dioxide) and a halogen atom is introduced its place. A catalytic approach has been developed. : History The reaction is named after Cläre Hunsdiecker and her husband Heinz Hunsdiecker, whose work in the 1930s developed it into a general method. The reaction was first demonstrated by Alexander Borodin in 1861 in his reports of the preparation of methyl bromide () from silver acetate (). Three decades later, Angelo Simonini, working as a student of Adolf Lieben at the University of Vienna, investigated the reactions of silver carboxylates with iodine. He found that the products formed are d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
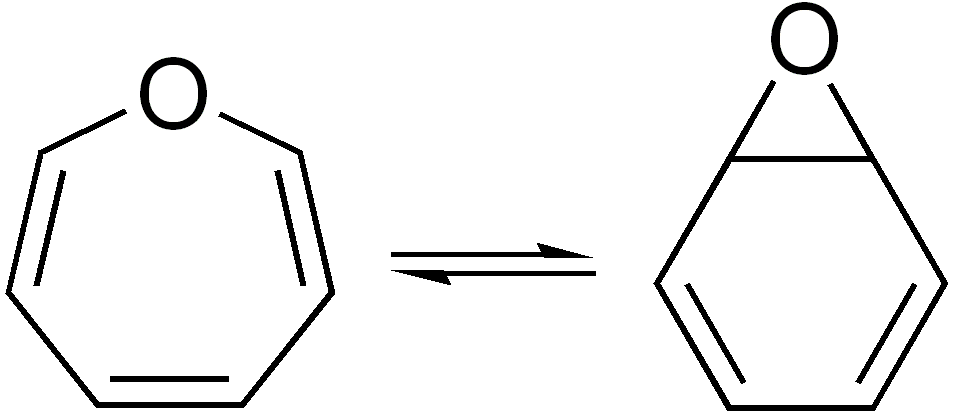
Valence Isomerization
In organic chemistry, two molecules are valence isomers when they are constitutional isomers that can interconvert through pericyclic reactions. Benzene There are many valence isomers one can draw for the C6H6 formula benzene. Some were originally proposed for benzene itself before the actual structure of benzene was known. Others were later synthesized in lab. Some have been observed to isomerize to benzene, whereas others tend to undergo other reactions instead, or isomerize by ways other than pericyclic reactions. Image:Benzene-2D-flat.png, Benzene Image:Historic Benzene Formulae Dewar(1867) V.1.svg, Dewar benzene Image:Prisman2.svg, Prismane Image:Benzvalene.png, Benzvalene Image:Bicycloprop-2-enyl.svg, Bicyclopropenyl Cyclooctatetraene The valence isomers are not restricted to isomers of benzene. Valence isomers are also seen in the series (CH)8. Due to the larger number of units, the number of possible valence isomers is also greater and at least 21: Image:Cyclooctate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heat Of Combustion
The heating value (or energy value or calorific value) of a substance, usually a fuel or food (see food energy), is the amount of heat released during the combustion of a specified amount of it. The ''calorific value'' is the total energy released as heat when a substance undergoes complete combustion with oxygen under standard conditions. The chemical reaction is typically a hydrocarbon or other organic molecule reacting with oxygen to form carbon dioxide and water and release heat. It may be expressed with the quantities: * energy/ mole of fuel * energy/mass of fuel * energy/volume of the fuel There are two kinds of enthalpy of combustion, called high(er) and low(er) heat(ing) value, depending on how much the products are allowed to cool and whether compounds like are allowed to condense. The high heat values are conventionally measured with a bomb calorimeter. Low heat values are calculated from high heat value test data. They may also be calculated as the difference be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strained Ring
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated. Ring strain results from a combination of angle strain, conformational strain or Pitzer strain (torsional eclipsing interactions), and transannular strain, also known as van der Waals strain or Prelog strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane. Ring strain energy can be attributed to the energy required for the distortion of bond and bond angles in order to close a ring. Ring strain energy is believed to be the cause of accelerated rates in altering ring reactions. Its interactions with traditional bond energies chan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kilocalorie Per Mole
The kilocalorie per mole is a unit to measure an amount of energy per number of molecules, atoms, or other similar particles. It is defined as one kilocalorie of energy (1000 thermochemical gram calories) per one mole of substance. The unit symbol is written kcal/mol or kcal⋅mol−1. As typically measured, one kcal/mol represents a temperature increase of one degree Celsius in one liter of water (with a mass of 1 kg) resulting from the reaction of one mole of reagents. In SI units, one kilocalorie per mole is equal to 4.184 kilojoules per mole (kJ/mol), which comes to approximately joules per molecule, or about 0.043 eV per molecule. At room temperature (25 °C, 77 °F, or 298.15 K), one kilocalorie per mole is approximately equal to 1.688 kT per molecule. Even though it is not an SI unit, the kilocalorie per mole is still widely used in chemistry and biology for thermodynamical quantities such as thermodynamic free energy, heat of vaporization, h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Free Energy Perturbation
Free-energy perturbation (FEP) is a method based on statistical mechanics that is used in computational chemistry for computing free-energy differences from molecular dynamics or Metropolis Monte Carlo simulations. The FEP method was introduced by Robert W. Zwanzig in 1954. According to the free-energy perturbation method, the free-energy difference for going from state A to state B is obtained from the following equation, known as the ''Zwanzig equation'': : \Delta F(\mathbf \to \mathbf) = F_\mathbf - F_\mathbf = -k_\text T \ln \left\langle \exp\left(-\frac \right) \right\rangle_\mathbf, where ''T'' is the temperature, ''k''B is the Boltzmann constant, and the angular brackets denote an average over a simulation run for state A. In practice, one runs a normal simulation for state A, but each time a new configuration is accepted, the energy for state B is also computed. The difference between states A and B may be in the atom types involved, in which case the Δ''F'' obtained ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Group
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula (whereas normal methane has the formula ). In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond (), it can be found on its own in any of three forms: methanide anion (), methylium cation () or methyl radical (). The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed. Methyl cation, anion, and radical Methyl cation The methylium cation () exists in the gas phase, but is otherwise not encountered. Some compounds are considered to be sources of the cation, and this simplification is used pervasively in organic chemistry. For ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial or biological significance, but more complex derivatives are important in biology and biotechnology. Structure The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. butane or cyclohexane. As such, cyclobutane is unstable above about 500 °C. The four carbon atoms in cyclobutane are not coplanar; instead, the ring typically adopts a folded or "puckered" conformation. This implies that the C-C-C angle is less than 90°. One of the carbon atoms makes a 25° angle with the plane formed by the other three carbons. In this way, some of the eclipsing interactions are reduced. The conformation is also known as a "butterfly". Equivalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring (chemistry)
In chemistry, a ring is an ambiguous term referring either to a simple cycle of atoms and bonds in a molecule or to a connected set of atoms and bonds in which every atom and bond is a member of a cycle (also called a ring system). A ring system that is a simple cycle is called a monocycle or simple ring, and one that is not a simple cycle is called a polycycle or polycyclic ring system. A simple ring contains the same number of sigma bonds as atoms, and a polycyclic ring system contains more sigma bonds than atoms. A molecule containing one or more rings is called a cyclic compound, and a molecule containing two or more rings (either in the same or different ring systems) is termed a polycyclic compound. A molecule containing no rings is called an acyclic or open-chain compound. Homocyclic and heterocyclic rings A homocycle or homocyclic ring is a ring in which all atoms are of the same chemical element. A heterocycle or heterocyclic ring is a ring containing atoms of at least ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring Expansion And Contraction
Ring expansion and ring contraction reactions expand or contract rings, usually in organic chemistry. The term usually refers to reactions involve making and breaking C-C bonds, Diverse pathways lead to these kinds of reactions. Many of these reactions are primarily of theoretical or pedagoogical interest, but some are very useful. Ring expansions Rings can be expanded by attack of the ring onto an outside group already appended to the ring (a migration/insertion), opening of a bicycle to a single larger ring, or coupling a ring closing with an expansion. These expansions can be further broken down by what type of atom they incorporate (a carbon or a heteroatom) into the expanded ring. Carbon insertion through migration to an exocyclic group These reactions have the general features of having an exocyclic leaving group on a carbon adjacent to the ring and an electron donating group on the ring capable of initiating a migration of an endocyclic bond. A common migration introd ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strain (chemistry)
In chemistry, a molecule experiences strain when its chemical structure undergoes some Stress (mechanics), stress which raises its internal energy in comparison to a strain-free reference Chemical compound, compound. The internal energy of a molecule consists of all the energy stored within it. A strained molecule has an additional amount of internal energy which an unstrained molecule does not. This extra internal energy, or strain energy, can be likened to a compression (physics), compressed spring (device), spring.Anslyn and Dougherty, ''Modern Physical Organic Chemistry'', University Science Books, 2006, Much like a compressed spring must be held in place to prevent release of its potential energy, a molecule can be held in an energetically unfavorable conformation by the Chemical bond, bonds within that molecule. Without the bonds holding the conformation in place, the strain energy would be released. Summary Thermodynamics The Chemical equilibrium, equilibrium of two c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |