|
Amylase Induced Fermentation
An amylase () is an enzyme that catalyses the hydrolysis of starch (Latin ') into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large amounts of starch but little sugar, such as rice and potatoes, may acquire a slightly sweet taste as they are chewed because amylase degrades some of their starch into sugar. The pancreas and salivary gland make amylase (alpha amylase) to hydrolyse dietary starch into disaccharides and trisaccharides which are converted by other enzymes to glucose to supply the body with energy. Plants and some bacteria also produce amylase. Specific amylase proteins are designated by different Greek letters. All amylases are glycoside hydrolases and act on α-1,4-glycosidic bonds. Classification α-Amylase The α-amylases () ( CAS 9014–71–5) (alternative names: 1,4-α-D-glucan glucanohydrolase; glycogenase) are calcium metalloenzymes. By acting at random ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossils of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name comes from Latin ''calx'' " lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharmaceuticals for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside Hydrolase Family 13
In molecular biology, glycoside hydrolase family 13 is a family of glycoside hydrolases. Glycoside hydrolases are a widespread group of enzymes that hydrolyse the glycosidic bond between two or more carbohydrates, or between a carbohydrate and a non-carbohydrate moiety. A classification system for glycoside hydrolases, based on sequence similarity, has led to the definition of >100 different families. This classification is available on the CAZy web site, and also discussed at CAZypedia, an online encyclopedia of carbohydrate active enzymes. Enzymes containing this domain belong to family 13CAZY GH_13 of the glycosyl hydrolases. The maltogenic alpha-amylase is an enzyme which catalyses hydrolysis of (1-4)-alpha-D-glucosidic linkages in polysaccharides so as to remove successive alpha-maltose residues from the non-reducing ends of the chains in the conversion of starch to maltose. Other enzymes in this family include neopullulanase, which hydrolyses pullulan to panose, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
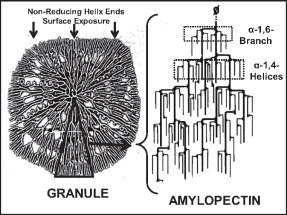
Amylopectin
Amylopectin is a water-insoluble polysaccharide and highly branched polymer of α-glucose units found in plants. It is one of the two components of starch, the other being amylose. Plants store starch within specialized organelles called amyloplasts. To generate energy, the plant hydrolyzes the starch, releasing the glucose subunits. Humans and other animals that eat plant foods also use amylase, an enzyme that assists in breaking down amylopectin, to initiate the hydrolysis of starch. Starch is made of about 70–80% amylopectin by weight, though it varies depending on the source. For example, it ranges from lower percent content in long-grain rice, amylomaize, and Russet Burbank potato, russet potatoes to 100% in glutinous rice, waxy potato starch, and waxy corn. Amylopectin is highly branched, being formed of 2,000 to 200,000 glucose units. Its inner chains are formed of 20–24 glucose subunits. Starch gelatinization, Dissolved amylopectin starch has a lower tendency of retrog ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amylose
Amylose is a polysaccharide made of α-D-glucose units, bonded to each other through α(1→4) glycosidic bonds. It is one of the two components of starch, making up approximately 20–25% of it. Because of its tightly packed Helix, helical structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch. Structure Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formula of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands. There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation, found both in starch granules and in hydrated amylose (when starch i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltose
} Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar. History Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. Its name comes from malt, combined with the suffix ' -ose' which is used in names of sugars. Structure and nomenclature Carbohydrates are generally divided into monosaccharides, oligosaccharides, and polysaccharides depending on th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltotriose
Maltotriose is a trisaccharide (three-part sugar) consisting of three glucose molecules linked with α-1,4 glycosidic bonds. It is most commonly produced by the digestive enzyme alpha-amylase (a common enzyme in human saliva) on amylose in starch. The creation of both maltotriose and maltose during this process is due to the random manner in which alpha amylase hydrolyses α-1,4 glycosidic bonds. It is the shortest chain oligosaccharide that can be classified as maltodextrin Maltodextrin is a name shared by two different families of chemicals. Both families are glucose polymers (also called ''dextrose polymers'' or ''Dextrin, dextrins''), but have little chemical or nutritional similarity. The digestible maltodextr .... References Trisaccharides Types of sugar {{Carbohydrate-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saccharides
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' may differ). This formula does not imply direct covalent bonding between hydrogen and oxygen atoms; for example, in , hydrogen is covalently bonded to carbon, not oxygen. While the 2:1 hydrogen-to-oxygen ratio is characteristic of many carbohydrates, exceptions exist. For instance, uronic acids and deoxy-sugars like fucose deviate from this precise stoichiometric definition. Conversely, some compounds conforming to this definition, such as formaldehyde and acetic acid, are not classified as carbohydrates. The term is predominantly used in biochemistry, functioning as a synonym for saccharide (), a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloprotein
Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins (out of ~20,000) contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins. Abundance It is estimated that approximately half of all proteins contain a metal. In another estimate, about one quarter to one third of all proteins are proposed to require metals to carry out their functions. Thus, metalloproteins have many different functions in cells, such as storage and transport of proteins, enzymes and signal transduction proteins, or infectious diseases. The abundance of metal binding proteins may be inherent to the amino acids that proteins use, as even artificial proteins without evolutionary history will readily bind metals. Most metals in the human body are bound to proteins. For instance, the relatively high concentration of iron in the human body ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight. It is used by plants to make cellulose, the most abundant carbohydrate in the world, for use in cell walls, and by all living Organism, organisms to make adenosine triphosphate (ATP), which is used by the cell as energy. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as amylose and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form is -glucose, while its Stereoisomerism, stereoisomer L-glucose, -glucose is produced synthetically in comparatively small amounts and is less biologicall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dextrin
Dextrins are a group of low-molecular-weight carbohydrates produced by the hydrolysis of starch and glycogen. Dextrins are mixtures of polymers of D-glucose units linked by α-(1→4) or α-(1→6) glycosidic bonds. Dextrins can be produced from starch using enzymes like amylases, as during digestion in the human body and during malting and mashing in beer brewing or by applying dry heat under acidic conditions (pyrolysis or roasting). This procedure was first discovered in 1811 by Edme-Jean Baptiste Bouillon-Lagrange. The latter process is used industrially, and also occurs on the surface of bread during the baking process, contributing to flavor, color and crispness. Dextrins produced by heat are also known as pyrodextrins. Starch hydrolyses during roasting under acidic conditions, and short-chained starch parts partially rebranch with α-(1,6) bonds to the degraded starch molecule. See also Maillard reaction. Dextrins are white, yellow, or brown powders that are partially o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |