|
Amide (functional Group)
In chemistry, the term amide ( or or ) is a compound with the functional group R''n''E(=O)''x''NR2, where ''n'' and ''x'' may be 1 or 2, E is some element, and each R represents an organic group or hydrogen. It is a derivative of an oxoacid R''n''E(=O)''x''OH with an hydroxy group –OH replaced by an amine group –NR2. Some important subclasses are * carboxamides, or organic amides, where E = carbon, with the general formula RC(=O)NR2. * phosphoramides, where E = phosphorus, such as R2P(=O)NR2 * sulfonamides, where E = sulfur, namely RS(=O)2NR2 The term amide may also refer to * amide group, a functional group –C(=O)N= consisting of a carbonyl adjacent to a nitrogen atom. * cyclic amide or lactam, a cyclic compound with the amide group –C(=O)N– in the ring. * metal amide, an ionic compound ("salt") with the azanide anion H2N− (the conjugate base of ammonia) or to a derivative thereof R2N−. There is also a neutral amino radical (•NH2) and a positively charg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amide Types
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, such as in the amino acids asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid () with the hydroxyl group () replaced by an amine group (); or, equivalently, an acyl (alkanoyl) group () joined to an amine group. Common examples of amides are acetamide (), benzamide (), and dimethylformamide (). Amides are qualified as primary, secondary, and tertiary according to whether the amine subgroup has the form , , or , where R and R' are groups other than hydrogen. The core of amides is called the amide group (specifically, carboxamide group). Amides are pervasive in nature and technology. Proteins and important plastics l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a carbonyl group is often referred to as a carbonyl compound. The term carbonyl can also refer to carbon monoxide as a ligand in an inorganic or organometallic complex (a metal carbonyl, e.g. nickel carbonyl). The remainder of this article concerns itself with the organic chemistry definition of carbonyl, where carbon and oxygen share a double bond. Carbonyl compounds In organic chemistry, a carbonyl group characterizes the following types of compounds: Other organic carbonyls are urea and the carbamates, the derivatives of acyl chlorides chloroformates and phosgene, carbonate esters, thioesters, lactones, lactams, hydroxamates, and isocyanates. Examples of inorganic carbonyl compounds are carbon dioxide and carbonyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrenium Ion
A nitrenium ion (also called: aminylium ion or imidonium ion (obsolete)) in organic chemistry is a reactive intermediate based on nitrogen with both an electron lone pair and a positive charge and with two substituents (). Nitrenium ions are isoelectronic with carbenes, and can exist in either a singlet or a triplet state. The parent nitrenium ion, , is a ground state triplet species with a gap of to the lowest energy singlet state. Conversely, most arylnitrenium ions are ground state singlets. Certain substituted arylnitrenium ions can be ground state triplets, however. Nitrenium ions can have microsecond or longer lifetimes in water. Aryl nitrenium ions are of biological interest because of their involvement in certain DNA damaging processes. They are generated upon ''in vivo'' oxidation of arylamines. The regiochemistry and energetics of the reaction of phenylnitrenium ion with guanine has been investigated using density functional theory computations. Nitrenium species ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Radical
In chemistry, the amino radical, , also known as the aminyl radical or azanyl radical, is the neutral form of the amide ion (). Aminyl radicals are highly reactive and consequently short-lived, like most radicals; however, they form an important part of nitrogen chemistry. In sufficiently high concentration, amino radicals dimerise to form hydrazine. While as a functional group is common in nature, forming a part of many compounds (e.g. the phenethylamines), the radical cannot be isolated in its free form. Synthesis Reaction 1: Formation of amino radical from ammonia Amino radicals can be produced by reacting OH radical with ammonia in irradiated aqueous solutions. This reaction is formulated as a hydrogen abstraction reaction. :NH3 + ^\mathbfOH -> ^\mathbfNH2 + H2O The rate constant (''k1'') for this reaction was determined to be , while the parallel reaction of OH with was found to be much slower. This rate was redetermined by using two-pulse radiolysis competition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate Base
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a hydrogen ion. On the other hand, a conjugate base is what is left over after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a species formed by the removal of a proton from an acid, as in the reverse reaction it is able to gain a hydrogen ion. Because some acids are capable of releasing multiple protons, the conjugate base of an acid may itself be acidic. In summary, this can be represented as the following chemical reaction: :acid + base conjugate\ base + conjugate\ acid Johannes Nicolaus Brønsted and Martin Lowry introduced the Brønsted–Lowry theory, which proposed that any compound that can transfer a proton to any other compound is an acid, and the compound that accepts the proton is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azanide
Azanide is the IUPAC-sanctioned name for the anion . The term is obscure; derivatives of are almost invariably referred to as amides, despite the fact that amide also refers to the organic functional group –. The anion is the conjugate base of ammonia, so it is formed by the self-ionization of ammonia. It is produced by deprotonation of ammonia, usually with strong bases or an alkali metal. Azanide has a H–N–H bond angle of 104.5°. Alkali metal derivatives The alkali metal derivatives are best known, although usually referred to as alkali metal amides. Examples include lithium amide, sodium amide, and potassium amide. These salt-like solids are produced by treating liquid ammonia with strong bases or directly with the alkali metals (blue liquid ammonia solutions due to the solvated electron): :, where M = Li, Na, K Silver(I) amide () is prepared similarly. Transition metal complexes of the amido ligand are often produced by salt metathesis reaction or by deprotonat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptides, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
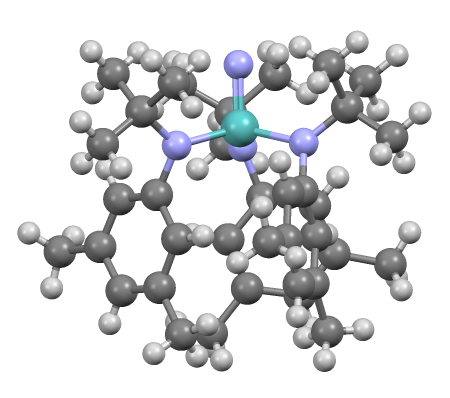
Metal Amide
Metal amides (systematic name metal azanides) are a class of coordination compounds composed of a metal center with amide ligands of the form NR2−. Amide ligands have two electron pairs available for bonding. In principle, they can be terminal or bridging. In these two examples, the dimethylamido ligands are both bridging and terminal: File:Tris(dimethylamino)aluminium dimer.png, Tris(dimethylamino)aluminium dimer File:Tris(dimethylamino)gallium dimer.png, Tris(dimethylamino)gallium dimer File:Ti(NMe2)4.png, Tetrakis(dimethylamino)titanium File:Ta(NMe2)5.png, Pentakis(dimethylamido)tantalum In practice, bulky amide ligands have a lesser tendency to bridge. Amide ligands may participate in metal-ligand π-bonding giving a complex with the metal center being co-planar with the nitrogen and substituents. Metal bis(trimethylsilyl)amides form a significant subcategory of metal amide compounds. These compounds tend to be discrete and soluble in organic solvents. Alkali metal amides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Compound
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present ( heterocyclic compounds). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactam
A lactam is a cyclic amide, formally derived from an amino alkanoic acid. The term is a portmanteau of the words ''lactone'' + ''amide''. Nomenclature Greek prefixes in alphabetical order indicate ring size: * α-Lactam (3-atom rings) * β-Lactam (4-atom rings) * γ-Lactam (5-atom rings) * δ-Lactam (6-atom rings) * ε-Lactam (7-atom rings) This ring-size nomenclature stems from the fact that a hydrolyzed α-Lactam leads to an α-amino acid and a β-Lactam to a β-amino acid, ''etc''. Synthesis General synthetic methods exist for the organic synthesis of lactams. Beckmann rearrangement Lactams form by the acid-catalyzed rearrangement of oximes in the Beckmann rearrangement. Schmidt reaction Lactams form from cyclic ketones and hydrazoic acid in the Schmidt reaction. Cyclization of amino acids Lactams can be formed from cyclisation of amino acids via the coupling between an amine and a carboxylic acid within the same molecule. Lactamization is most efficient in this ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many industrially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

-3D-balls.png)