|
Aluminium Silicate
Aluminum silicate (or aluminium silicate) is a name commonly applied to chemical compounds which are derived from aluminium oxide, Al2O3 and silicon dioxide, SiO2 which may be anhydrous or hydrated, naturally occurring as minerals or synthetic. Their chemical formulae are often expressed as xAl2O3·ySiO2·zH2O. It is known as E number E559. Main representatives Andalusite, kyanite, and sillimanite are the principal aluminium silicate minerals. The triple point of the three polymorphs is located at a temperature of and a pressure of . These three minerals are commonly used as index minerals in metamorphic rocks. * Al2SiO5, (Al2O3·SiO2), which occurs naturally as the minerals andalusite, kyanite and sillimanite which have distinct crystal structures. * Al2Si2O7, (Al2O3·2SiO2), called metakaolinite, formed from kaolin by heating at . * Al6Si2O13, (3Al2O3·2SiO2), the mineral mullite, the only thermodynamically stable intermediate phase in the Al2O3-SiO2 system at at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Oxide
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several Aluminium oxide (compounds), aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, ALOX or alundum in various forms and applications and alumina is refined from bauxite. It occurs naturally in its crystalline Polymorphism (materials science), polymorphic phase (matter), phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire,which have an alumina content approaching 100%. Al2O3 is used as feedstock to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point. Natural occurrence Corundum is the most common naturally occurring crystallinity, crystalline form of aluminium oxide. ruby, Rubies and sapphires are gem-quality forms o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metamorphic Rock
Metamorphic rocks arise from the transformation of existing rock to new types of rock in a process called metamorphism. The original rock ( protolith) is subjected to temperatures greater than and, often, elevated pressure of or more, causing profound physical or chemical changes. During this process, the rock remains mostly in the solid state, but gradually recrystallizes to a new texture or mineral composition. The protolith may be an igneous, sedimentary, or existing metamorphic rock. Metamorphic rocks make up a large part of the Earth's crust and form 12% of the Earth's land surface. They are classified by their protolith, their chemical and mineral makeup, and their texture. They may be formed simply by being deeply buried beneath the Earth's surface, where they are subject to high temperatures and the great pressure of the rock layers above. They can also form from tectonic processes such as continental collisions, which cause horizontal pressure, friction, and dist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
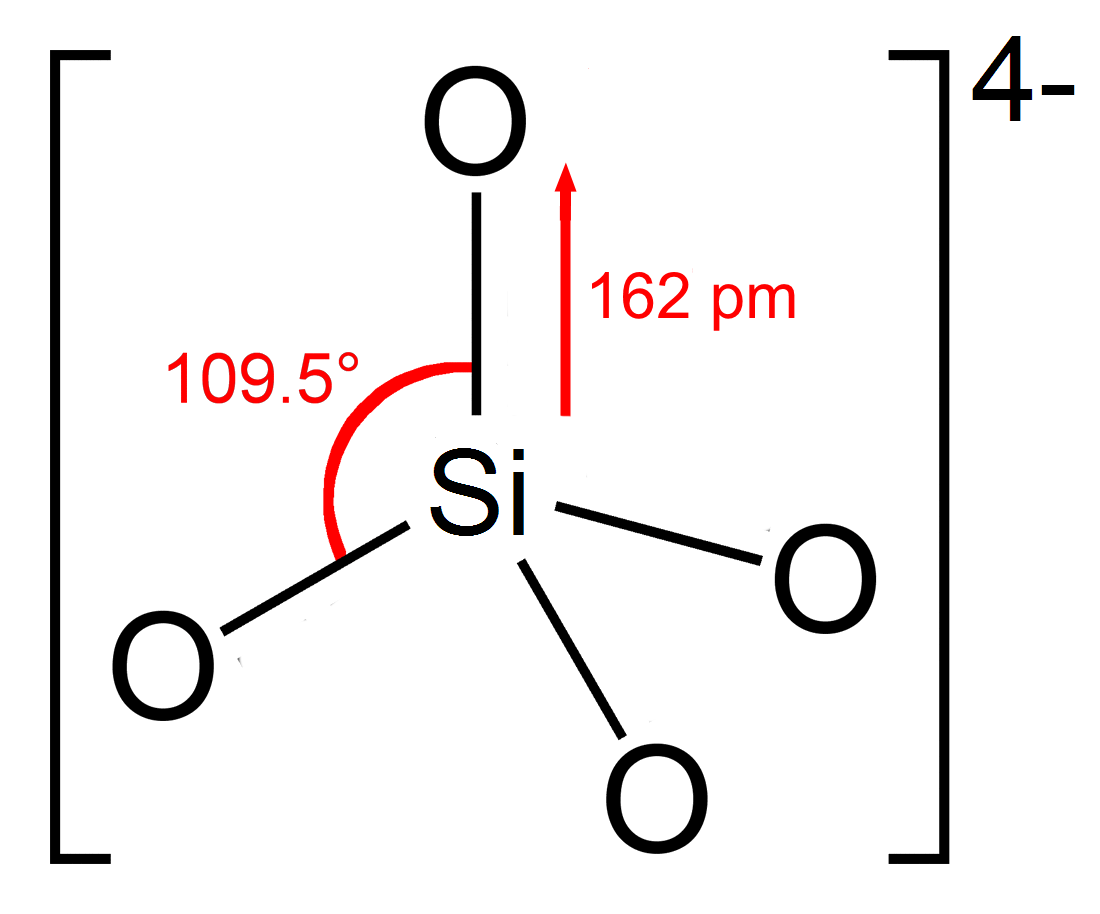
Silicates
A silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt (chemistry), salt of such anions, such as sodium metasilicate; or any ester containing the corresponding Moiety (chemistry), chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicic acid, hexafluorosilicate . Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In most silicates, a silicon atom occupies th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Compounds
Aluminium (British and IUPAC spellings) or aluminum ( North American spelling) combines characteristics of pre- and post-transition metals. Since it has few available electrons for metallic bonding, like its heavier group 13 congeners, it has the characteristic physical properties of a post-transition metal, with longer-than-expected interatomic distances.Greenwood and Earnshaw, pp. 222–4 Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and aluminium compounds tend towards covalency;Greenwood and Earnshaw, pp. 224–7 this behaviour is similar to that of beryllium (Be2+), an example of a diagonal relationship.Greenwood and Earnshaw, pp. 112–3 However, unlike all other post-transition metals, the underlying core under aluminium's valence shell is that of the preceding noble gas, whereas for gallium and indium it is that of the preceding noble gas plus a filled d-subshell, and for thallium and nihonium it is that of the preceding noble gas plus f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows Tetrahedral molecular geometry, tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alumina
Aluminium oxide (or aluminium(III) oxide) is a chemical compound of aluminium and oxygen with the chemical formula . It is the most commonly occurring of several aluminium oxides, and specifically identified as aluminium oxide. It is commonly called alumina and may also be called aloxide, aloxite, ALOX or alundum in various forms and applications and alumina is refined from bauxite. It occurs naturally in its crystalline polymorphic phase α-Al2O3 as the mineral corundum, varieties of which form the precious gemstones ruby and sapphire,which have an alumina content approaching 100%. Al2O3 is used as feedstock to produce aluminium metal, as an abrasive owing to its hardness, and as a refractory material owing to its high melting point. Natural occurrence Corundum is the most common naturally occurring crystalline form of aluminium oxide. Rubies and sapphires are gem-quality forms of corundum, which owe their characteristic colours to trace impurities. Rubies are given their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kaolinite
Kaolinite ( ; also called kaolin) is a clay mineral, with the chemical composition Al2 Si2 O5( OH)4. It is a layered silicate mineral, with one tetrahedral sheet of silica () linked through oxygen atoms to one octahedral sheet of alumina (). Kaolinite is a soft, earthy, usually white, mineral (dioctahedral phyllosilicate clay), produced by the chemical weathering of aluminium silicate minerals like feldspar. It has a low shrink–swell capacity and a low cation-exchange capacity (1–15 meq/100 g). Rocks that are rich in kaolinite, and halloysite, are known as kaolin () or china clay. In many parts of the world kaolin is colored pink-orange-red by iron oxide, giving it a distinct rust hue. Lower concentrations of iron oxide yield the white, yellow, or light orange colors of kaolin. Alternating lighter and darker layers are sometimes found, as at Providence Canyon State Park in Georgia, United States. Kaolin is an important raw material in many industries and app ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
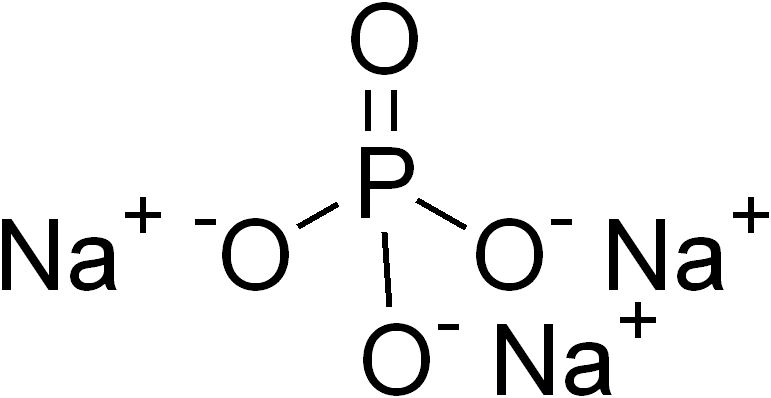
Ternary Phase
In inorganic chemistry and materials chemistry, a ternary compound or ternary phase is a chemical compound containing three different elements. While some ternary compounds are molecular, ''e.g.'' chloroform (), more typically ternary phases refer to extended solids. The perovskites are a famous example. Binary phases, with only two elements, have lower degrees of complexity than ternary phases. With four elements, quaternary phases are more complex. The number of isomers of a ternary compound provide a distinction between inorganic and organic chemistry: "In inorganic chemistry one or, at most, only a few compounds composed of any two or three elements were known, whereas in organic chemistry the situation was very different." Ternary crystalline compounds An example is sodium phosphate, . The sodium ion has a charge of 1+ and the phosphate ion has a charge of 3–. Therefore, three sodium ions are needed to balance the charge of one phosphate ion. Another example of a te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mullite
Mullite or porcelainite is a rare silicate mineral formed during contact metamorphism of clay minerals. It can form two stoichiometric forms: 3 Al2 O32 SiO2 or 2Al2O3 SiO2. Unusually, mullite has no charge-balancing cations present. As a result, there are three different aluminium sites: two distorted tetrahedral and one octahedral. Mullite was first described in 1924 for an occurrence on the Isle of Mull, Scotland. It occurs as argillaceous inclusions in volcanic rocks in the Isle of Mull, inclusions in sillimanite within a tonalite at Val Sissone, Italy and with emerylike rocks in Argyllshire, Scotland. Porcellanite Mullite (porcelainite) can be found as a constituent mineral in a type of thermally-metamorphosed rock called porcellanite. Use in porcelain Mullite is present in the form of needles in porcelain. It is produced during various melting and firing processes, and is used as a refractory material,H. Schneider & S. Komarneni (2005) Mullite. Wiley, VCH, 5 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Andalusite
Andalusite is an aluminium nesosilicate mineral with the chemical formula Al2SiO5. This mineral was called andalousite by Delamétherie, who thought it came from Andalusia, Spain. It soon became clear that it was a locality error, and that the specimens studied were actually from El Cardoso de la Sierra, in the Spanish province of Guadalajara, not Andalusia. Andalusite is trimorphic with kyanite and sillimanite, being the lower pressure mid temperature polymorph. At higher temperatures and pressures, andalusite may convert to sillimanite. Thus, as with its other polymorphs, andalusite is an aluminosilicate index mineral, providing clues to depth and pressures involved in producing the host rock. Varieties The variety chiastolite commonly contains dark inclusions of carbon or clay which form a cruciform pattern when shown in cross-section. This stone was known at least from the sixteenth century, being taken to many European countries, as a souvenir, by pilgrims returning fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Index Mineral
An index mineral is used in geology to determine the degree of metamorphism a rock has experienced. Depending on the original composition of and the pressure and temperature experienced by the protolith (parent rock), chemical reactions between minerals in the solid state produce new minerals. When an index mineral is found in a metamorphosed rock, it indicates the minimum pressure and temperature the protolith must have achieved in order for that mineral to form. The higher the pressure and temperature in which the rock formed, the higher the grade of the rock. The concept traces its roots to 1912, when G. M. Barrow mapped zones of metamorphism in Scotland. Each zone is named for the index mineral that appears in it, for example, the chlorite zone is named for chlorite. Mineralogic zones Mudrock, a fine-grained sedimentary rock often containing aluminium-rich minerals, produces these minerals after being metamorphosed, from low to high grade:Blatt, Harvey and Robert J. Tracy, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon Dioxide
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |