|
AlInGaP
Aluminium gallium indium phosphide (, also AlInGaP, InGaAlP, etc.) is a semiconductor material that provides a platform for the development of Multi-junction solar cell, multi-junction photovoltaics and optoelectronic devices. It has a direct and indirect band gaps, direct bandgap ranging from ultraviolet to infrared photon energies. AlGaInP is used in heterostructures for high-brightness red, orange, green, and yellow light-emitting diodes. It is also used to make diode lasers. Preparation AlGaInP is typically grown by heteroepitaxy on gallium arsenide or gallium phosphide substrates in order to form a quantum well structure that can be fabricated into different devices. Properties The direct bandgap of AlGaInP encompasses the energy range of visible light (1.7 eV - 3.1 eV). By selecting a specific composition of AlGaInP, the bandgap can be selected to correspond to the energy of a specific wavelength of visible light. For instance, this can be used to obtain LEDs th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
III-V Semiconductors
Semiconductor materials are nominally small band gap insulators. The defining property of a semiconductor material is that it can be compromised by doping it with impurities that alter its electronic properties in a controllable way. Because of their application in the computer and photovoltaic industry—in devices such as transistors, lasers, and solar cells—the search for new semiconductor materials and the improvement of existing materials is an important field of study in materials science. Most commonly used semiconductor materials are crystalline inorganic solids. These materials are classified according to the periodic table groups of their constituent atoms. Different semiconductor materials differ in their properties. Thus, in comparison with silicon, compound semiconductors have both advantages and disadvantages. For example, gallium arsenide (GaAs) has six times higher electron mobility than silicon, which allows faster operation; wider band gap, which allows op ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Crystal System
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc) Note: the term fcc is often used in synonym for the ''cubic close-packed'' or ccp structure occurring in metals. However, fcc stands for a face-centered cubic Bravais lattice, which is not necessarily close-packed when a motif is set onto the lattice points. E.g. the diamond and the zincblende lattices are fcc but not close-packed. Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais latices ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
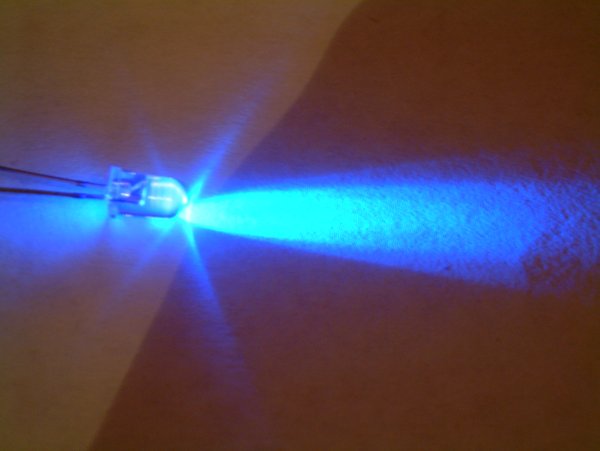
Indium Gallium Nitride
Indium gallium nitride (InGaN, ) is a semiconductor material made of a mix of gallium nitride (GaN) and indium nitride (InN). It is a ternary group III/ group V direct bandgap semiconductor. Its bandgap can be tuned by varying the amount of indium in the alloy. InxGa1−xN has a direct bandgap span from the infrared (0.69 eV) for InN to the ultraviolet (3.4 eV) of GaN. The ratio of In/Ga is usually between 0.02/0.98 and 0.3/0.7. Applications LEDs Indium gallium nitride is the light-emitting layer in modern blue and green LEDs and often grown on a GaN buffer on a transparent substrate as, e.g. sapphire or silicon carbide. It has a high heat capacity and its sensitivity to ionizing radiation is low (like other group III nitrides), making it also a potentially suitable material for solar photovoltaic devices, specifically for arrays for satellites. It is theoretically predicted that spinodal decomposition of indium nitride should occur for compositions between 15% and 85%, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Compounds
Indium is a chemical element; it has symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are largely intermediate between the two. It was discovered in 1863 by Ferdinand Reich and Hieronymous Theodor Richter by spectroscopic methods and named for the indigo blue line in its spectrum. Indium is used primarily in the production of flat-panel displays as indium tin oxide (ITO), a transparent and conductive coating applied to glass. It is also used in the semiconductor industry, in low-melting-point metal alloys such as solders and soft-metal high-vacuum seals. It is produced exclusively as a by-product during the processing of the ores of other metals, chiefly from sphalerite and other zinc sulfide ores. Indium has no biological role and its compounds are toxic when inhaled or injected into the bloodstream, although they are poorly absorbed foll ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Compounds
Gallium compounds are compounds containing the element gallium. These compounds are found primarily in the +3 oxidation state. The +1 oxidation state is also found in some compounds, although it is less common than it is for gallium's heavier congeners indium and thallium. For example, the very stable GaCl2 contains both gallium(I) and gallium(III) and can be formulated as GaIGaIIICl4; in contrast, the monochloride is unstable above 0 °C, disproportionating into elemental gallium and gallium(III) chloride. Compounds containing Ga–Ga bonds are true gallium(II) compounds, such as GaS (which can be formulated as Ga24+(S2−)2) and the dioxan complex Ga2Cl4(C4H8O2)2.Greenwood and Earnshaw, p. 240 There are also compounds of gallium with negative oxidation states, ranging from −5 to −1, most of these compounds being magnesium gallides (MgxGay). Aqueous chemistry Strong acids dissolve gallium, forming gallium(III) salts such as (gallium nitrate). Aqueous solutions o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Compounds
Aluminium (British and IUPAC spellings) or aluminum ( North American spelling) combines characteristics of pre- and post-transition metals. Since it has few available electrons for metallic bonding, like its heavier group 13 congeners, it has the characteristic physical properties of a post-transition metal, with longer-than-expected interatomic distances.Greenwood and Earnshaw, pp. 222–4 Furthermore, as Al3+ is a small and highly charged cation, it is strongly polarizing and aluminium compounds tend towards covalency;Greenwood and Earnshaw, pp. 224–7 this behaviour is similar to that of beryllium (Be2+), an example of a diagonal relationship.Greenwood and Earnshaw, pp. 112–3 However, unlike all other post-transition metals, the underlying core under aluminium's valence shell is that of the preceding noble gas, whereas for gallium and indium it is that of the preceding noble gas plus a filled d-subshell, and for thallium and nihonium it is that of the preceding noble gas plus f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Gallium Arsenide Phosphide
Indium gallium arsenide phosphide () is a quaternary compound semiconductor material, an alloy of gallium arsenide, gallium phosphide, indium arsenide, or indium phosphide. This compound has applications in photonic devices, due to the ability to tailor its band gap via changes in the alloy mole ratios, ''x'' and ''y''. Indium phosphide-based photonic integrated circuits, or PICs, commonly use alloys of to construct quantum wells, waveguides and other photonic structures, lattice matched to an InP substrate, enabling single-crystal epitaxial growth onto InP. Many devices operating in the near-infrared 1.55 μm wavelength window utilize this alloy, and are employed as optical components (such as laser transmitters, photodetectors and modulators) in C-band communications systems. Fraunhofer Institute for Solar Energy Systems ISE reported a triple-junction solar cell utilizing . The cell has very high efficiency of 35.9% (claimed to be a record). See also * Indium gallium ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Gallium Phosphide
Aluminium gallium phosphide, , a phosphide of aluminium and gallium, is a semiconductor material. It is an alloy of aluminium phosphide and gallium phosphide. It is used to manufacture light-emitting diode A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corre ...s emitting green light. See also * Aluminium gallium indium phosphide External linksLight-Emitting Diode - An Introduction, Structure, and Applications of LEDs Aluminium compounds Gallium compounds Phosphides III-V semiconductors III-V compounds Zincblende crystal structure {{CMP-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Gallium Phosphide
Indium gallium phosphide (InGaP), also called gallium indium phosphide (GaInP), is a semiconductor composed of indium, gallium and phosphorus. It is used in high-power and high-frequency electronics because of its superior electron velocity with respect to the more common semiconductors silicon and gallium arsenide. It is used mainly in HEMT and HBT structures, but also for the fabrication of high efficiency solar cells used for space applications and, in combination with aluminium ( AlGaInP alloy) to make high brightness LEDs with orange-red, orange, yellow, and green colors. Some semiconductor devices such as EFluor Nanocrystal use InGaP as their core particle. Indium gallium phosphide is a solid solution of indium phosphide and gallium phosphide. Ga0.5In0.5P is a solid solution of special importance, which is almost lattice matched to GaAs. This allows, in combination with (AlxGa1−x)0.5In0.5, the growth of lattice matched quantum wells for red emitting semiconductor las ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Phosphide
Indium phosphide (InP) is a binary semiconductor composed of indium and phosphorus. It has a face-centered cubic ("zincblende (crystal structure), zincblende") crystal structure, identical to that of gallium arsenide, GaAs and most of the List of semiconductor materials, III-V semiconductors. Manufacturing Indium phosphide can be prepared from the reaction of white phosphorus and indium iodide at 400 °C., also by direct combination of the purified elements at high temperature and pressure, or by thermal decomposition of a mixture of a trialkyl indium compound and phosphine. Applications The application fields of InP splits up into three main areas. It is used as the basis for optoelectronic components, high-speed electronics, and photovoltaics High-speed optoelectronics InP is used as a substrate for epitaxy, epitaxial optoelectronic devices based other semiconductors, such as indium gallium arsenide. The devices include pseudomorphic heterojunction bipolar transistors th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MOVPE
Metalorganic vapour-phase epitaxy (MOVPE), also known as organometallic vapour-phase epitaxy (OMVPE) or metalorganic chemical vapour deposition (MOCVD), is a chemical vapour deposition method used to produce single- or polycrystalline thin films. It is a process for growing crystalline layers to create complex semiconductor multilayer structures. In contrast to molecular-beam epitaxy (MBE), the growth of crystals is by chemical reaction and not physical deposition. This takes place not in vacuum, but from the gas phase at moderate pressures (10 to 760 Torr). As such, this technique is preferred for the formation of devices incorporating thermodynamically metastable alloys, and it has become a major process in the manufacture of optoelectronics, such as light-emitting diodes, its most widespread application. It was first demonstrated in 1967 at North American Aviation (later Rockwell International) Autonetics Division in Anaheim CA by Harold M. Manasevit. Basic principl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine () is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764–1838), a student of Lavoisi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |